Atom (science)
An atom (from the Greek atomos, indivisible)[1] [2] is the smallest physical entity (particle) that can represent a chemical element—that retains the properties of a sensible/tangible piece of a chemical element—elements which nature has produced more than 90 different species (e.g., copper, gold, aluminium), each characterized by a species-specific number of protons in their atoms' nuclei, and by a species-variable number of atom varieties (called isotopes), each isotope characterized by an isotope-specific number of neutrons in their atoms' nuclei. Atoms of the chemical element, carbon, for example, each contain six protons in their nuclei, but differing numbers of neutrons, most carbon atoms containing six neutrons, though about 1% of them contain seven neutrons, and tiny fractions of about a dozen other varieties of carbon atoms contain fewer and greater numbers of neutrons than 6-7.[3]
If, in some cataclysm, all of scientific knowledge were to be destroyed, and only one sentence passed on to the next generations of creatures, what statement would contain the most information in the fewest words? I believe it is the atomic hypothesis (or the atomic fact, or whatever you wish to call it) that all things are made of atoms—litte particles that move around in perpetual motion, attracting each other when they are a little distance apart, but repelling upon being squeezed into one another. In that one sentence, you will see, there is an enormous amount of information about the world, if just a little imagination and thinking are applied.
|
Atoms serve as the building blocks of all the matter that we come in contact with on a daily basis, solids, liquids and gases; matter composed of a single or assorted combinations of chemical elements; matter that can undergo spontaneous chemical change; matter that chemists can, through chemical reactions, manipulate to produce all of the synthetic products that humans use.
Those facts and potentialities derive from the atomic hypothesis, first proposed by the ancient Greeks (5th century BCE: Leucippus[5], Democritus[6]) and first established on an experimental and quantitative basis in the early 1800s by the English scientist, John Dalton.
Individually atoms are astonishingly small (carbon atom diameter: ~2.2•10–8cm = 22 nm[7], or about 10,000 to 100,000 times smaller than a grain of sand—diameter of a grain of sand: between 2•10-3 cm = 20,000 nm and 2•10-2 cm = 200,000 nm[8]). Accordingly, it takes immense numbers of them to form most objects with which we are familiar. An average adult human body, for example, contains an estimated 7•1027 atoms.[9]
Atoms were once thought to be the fundamental building blocks of the entire universe. We now know, however, that atoms are made up of a few smaller subatomic-particles, the same set of which, in differing combinations, make up all the different chemical elements. Many of the chemical elements have non-stable, so-called radioactive isotopes, whose atomic nuclei emit matter and energy.
|
|
|
Introduction
It would be a poor thing to be an atom in a universe without physicists, and physicists are made up of atoms. A physicist is an atom's way of knowing about atoms.
|
The ancient Greek (5th century BCE[5] [6]) idea that all matter in the universe consisted of tiny indivisible entities had no empirical support at the time. Much later the English polymaths, Robert Boyle (dates) and Isaac Newton (1643-1727) championed the idea and added to the argument—as "corpuscularism".
The search for the most fundamental entities in the universe has remained one of most important driving forces in science to this day.
The particles that today bear the name "atom" were first encountered by early chemists who discovered that they could not reduce them to a more 'elementary' substance by chemical methods.
The modern atom serves as the fundamental particle for chemistry and as the smallest stable structure for engineering. Nuclear energy is generated either by breaking an atom into two or more smaller atoms (fission), or by combining two smaller atoms into a larger one (fusion).
History of the Atom
The nineteenth century
In the beginning of the nineteenth century men such as John Dalton, Joseph-Louis Gay-Lussac and Amedeo Avogadro hypothesized that matter consists of minute particles, atoms as Dalton liked to call them, or molecules (a term preferred by Avogadro and already used by Lavoisier). However, the difference between the two concepts was not clear. One of the main topics of the first international chemistry conference, the historic 1860 Karlsruhe conference, was to clear up the confusion about the difference between atoms and molecules. Stanislao Cannizaro offered for the first time in the history of science, a very clear definition of atoms as distinguished from molecules. To him the atom was the smallest quantity of each element which enters as a whole into the molecules which contain it. His statements represented a most remarkable contribution to the clarification of the issues debated at the time concerning the relations between atoms and molecules in both organic and inorganic compounds.
Albert Einstein gives molecules a reality in his explanation of Brownian motion
Employing the kinetic theory of heat and a mathematical approach to changes in velocity characterized by random motion, in 1905 Albert Einstein gave a convincing account of the microscopically observable random motion of pollen particles suspended in a liquid, previously noted by the botanist Robert Brown, attributing the motion to random collisions of the pollen particles with the molecules of the liquid in thermal motion. [11]
J.J. Thomson's "Plum Pudding" Model
The first widely accepted model of the atom was J.J Thomson's Model of a positively charged cloud with negatively charged "corpuscles," electrons, interspersed throughout. Thomson proposed and searched for configurations for which these particles had normal modes of vibration and were stable. He built his model using only electrostatic forces, which required that the electrons be in constant motion, this led to further difficulties and he was never able to create a model that matched observed data.
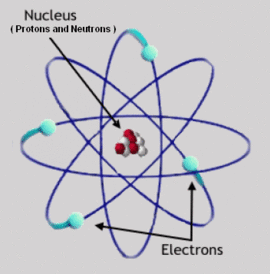
Rutherford Model
Ernest Rutherford, one of Thomson's students, disproved his theory in his now famous scattering experiment by showing that there was a dense, positively charged core at the center of each atom. Rutherford postulated that the structure of an atom more closely resembled a solar system with the nucleus at the center and electrons orbiting around it. Unfortunately, Classical Mechanics predicts that these orbits would be unstable, they would decay in less than a microsecond, while emitting a continuous spectrum of light. However, it was already known that each element emitted a unique spectrum of discrete lines. Rutherford could not explain why these orbits did not decay nor why atoms did not emit the continuous spectrum that these decays would have caused.
Bohr's model of the Hydrogen Atom
In 1913 Niels Bohr[12] devised the first quantum mechanical model of a (one-electron) atom.[13] He resolved the difficulties of Rutherford's model by making the non-classical assumption that there were only discrete and stationary energy states allowed for the electron. Further, Bohr conjectured that the angular momentum of each electron was constrained to be a positive integer times Planck's constant h divided by 2π. Since in Bohr's model electrons can only make jumps between the stationary states, the atomic spectral lines were explained by the discreteness of the energy differences of the states. Emission of a photon occurs when an electron falls down from a state with energy E2 to a state with lower energy E1. The frequency ν of the emitted photon is given by
Absorption of a photon of energy hν occurs when hν "matches" an atomic energy difference. An atom in a certain state jumps to a state of higher energy such that the energy difference is equal to hν. Clearly, this requirement puts a strict constraint on the frequencies of the photons that may be absorbed by the atom.
Bohr's theory was for one-electron atoms only, but he conjectured that many-electron atoms would have a similar structure. The extension of his theory to many-electron systems led a decade later to Heisenberg's matrix mechanics and Schrödinger's wave mechanics.
Today's view
For most practical purposes Bohr's model has proven an acceptable approximation. However, the quantum mechanical interpretation is that the electrons are spread out in various probability distributions around the nucleus rather than actually orbiting it.
Structure of the Atom
Atoms are made of a dense nucleus formed by combinations of the two nucleons (positively charged protons and zero charge neutrons) and surrounded by a much larger "cloud" of electrons. The vast majority of the volume of an atom is empty space. The number of protons contained in the nucleus determines the atomic number and in turn which element it is classified as. The number of neutrons further specifies the isotope number of that element. The number of electrons surrounding the nucleus is typically assumed to be equal to the number of protons in order to keep the entire atom electrically neutral. Atoms that are not neutral are called ions, they are designated by their charge in units of elementary charge, which is equal to the negative of the number of surplus electrons present around the atom. Thus an atom with one extra electron is charged -1, and one missing is charged +1.
Forces in the atom
The nucleus of the atom contains a high concentration of positively charged particles with no counter balancing negatively charged particles to keep it stable. In order to explain the existence of the nucleus scientists introduced the two nuclear forces, the strong force and the weak force. We now understand that the nucleus is held together by the residual strong force despite its significant positive charge. The electrons which surround the atom are electro-statically attracted to the nucleus due to their negative charge.
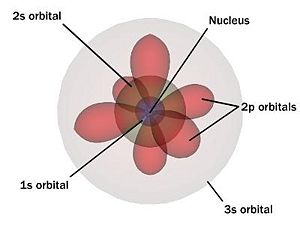
Electron Quantum States
Electrons surround the nucleus of an atom, where each is in a unique quantum mechanical state in the atom's structure. These states of well defined energy are called atomic orbitals and are organized from the lowest energy to highest. Each orbit can be uniquely defined by four quantum numbers: 'n' the shell, 'l' the angular momentum, 'm' the magnetic angular momentum, and 's' the spin. Shell numbers can only be natural numbers but no shell number over 7 has ever been observed. Each shell has n² spatial orbitals which are grouped by angular momentum 'l' into subshells. These subshells are often given the names 's', 'p', 'f', 'g', and 'h' rather than numerals. Each of these subshells contains ((2*l)+1) suborbitals 'm' which are numbered (−l...,0,...l). Finally, each suborbital can contain two electrons of different spin and . For a total of 2n² electrons per shell.
The valence, or the highest energy shell which contains electrons, is responsible for most of an elements chemical properties.
An atom with electrons in higher energy states without first filling lower states is considered "excited" and is likely to emit this excess energy in the form of a photon of energy equal to the difference between the current state and a lower energy state until it is no longer excited. These emissions are responsible for the emission and absorption spectra that atoms produce. In fact it is this property that allows us to discern the composition of stars and planets from a great distance.
Nuclei Quantum States
The nucleus of an atom itself also has a shell like behavior not dissimilar to electron shells. This leads to some extra stable and semi-stable states when the number of protons or neutrons in the nucleus is equal to the "magic numbers" 20, 28, 50, 82, and 126 which is believed to correspond to complete shells. [14] Stable nuclei fall upon a "line" of stability which starts very close to equal numbers of protons and neutrons and move towards favoring neutrons for heavier nuclei. This is a result of the fact that the electrostatic repulsion of two protons drops in magnitude much more slowly than the residual strong force. Either way nucleons strongly prefer to bond in even numbers.
Decay
Most combinations of nucleons are inherently unstable and undergo a number of radioactive decays in order to form more stable nuclei. In all of the common decays a particle is emitted from the nucleus in order to compensate for some instability in the atom.
- Alpha decay most frequently occurs in atoms which are simply too big, atoms are limited in size because the residual strong force which holds them together only acts over very small distances so that the rate of electrostatic repulsion grows faster than the rate of strong attraction as the nucleus grows. Alpha decay emits an Alpha particle which is denoted with the Greek letter α.
- Beta+ decay occurs in atoms which are proton heavy. In this decay a proton decays into a neutron and emits both a positron and a neutrino. The only natural Beta+ emitter is 40K.
- Beta- decay occurs in atoms which are neutron heavy. In this decay a neutron decays into a proton and emits both an electron and a neutrino. A lone proton can beta- decay.
- Gamma decay occurs in atoms where the nucleus is in an excited state. The nucleus will turn this excess energy into a high energy photon, typically called a gamma ray. Gamma decays typically follow either an alpha or beta decay which leaves the nucleus with excess energy.
- Electron capture occurs when a proton inside the nucleus captures an electron and becomes a neutron.
References
- ↑ http://www.webster.com/dictionary/atom
- ↑ Word-Origins.
- ↑ Carbon Technical Data.
- ↑ Feynman FP. (1963, 1989, 1995, 2011) Six Easy Pieces: Essentials of Physics Explained by Its Most Brilliant Teacher. With Robert B. Leighton and Matthew Sands. Introduction by Paul Davies. Quote from: Chapter 1. Title: Atoms in Motion. Section: Matter is made of atoms. Philadelphia: Basic Books, Perseus Books Group. Kindle edition. eISBN 978-0-465-02529-9
- ↑ Jump up to: 5.0 5.1 Leucippus. Stanford Encyclopedia of Philosophy.
- ↑ Jump up to: 6.0 6.1 Democritus. Stanford Encyclopedia of Philosophy.
- ↑ The size of atoms. Hyperphysics.
- ↑ Diameter of a Grain of Sand. The Physic Fact Book. See website for source-citations.
- ↑ How many atoms are there in the human body? Thomas Jefferson National Accelerator Facility.
- ↑ Warren WS. (2000) The physical basis of chemistry. 2nd edition. Academic Press. George Wald quote, p. xi.
- ↑ Renn J. (2005) Einstein's invention of Brownian motion. Ann. Phys. (Leipzig) 14, Supplement, 23-37. | Google Books preview of Einstein's Annalen papers: the complete collection 1901-1922, Volume 14 of Annalen der Physik. Jürgen Renn (editor).
- ↑ N. Bohr, Philosophical Magazine 26, p. 1 (1913)
- ↑ Fowler M. The Bohr Atom. Article in : Modern Physics Website, Michael Fowler, University of Virginia.
- Sections: Bohr Comes to Cambridge | What Determines Atomic Size? | Nicolson: a Clever Idea about a Wrong Model | Bohr Returns to Denmark | Bohr Changes his Mind about Spectra | Bohr Finds the Rydberg Constant without Doing an Experiment | Derivation of the Angular Momentum Quantization from the Correspondence Principle.
- ↑ Paul A. Tipler, Ralph A. Llewellyn (2003), Modern Physics ISBN 0716743450