Coulomb's law: Difference between revisions
imported>Paul Wormer |
imported>Paul Wormer |
||
| Line 13: | Line 13: | ||
The quantities ε<sub>0</sub> and ε<sub>''r''</sub> are the [[vacuum permittivity]] and the relative static permittivity (also known as relative dielectric constant) of the medium, respectively. The relative dielectric constant of air is: ε<sub>''r''</sub> = 1.000536 (at ambient temperature and pressure). The formulation of Coulomb's law is in the rationalized [[SI]] system of units. | The quantities ε<sub>0</sub> and ε<sub>''r''</sub> are the [[vacuum permittivity]] and the relative static permittivity (also known as relative dielectric constant) of the medium, respectively. The relative dielectric constant of air is: ε<sub>''r''</sub> = 1.000536 (at ambient temperature and pressure). The formulation of Coulomb's law is in the rationalized [[SI]] system of units. | ||
== | ==Electrostatic vector field== | ||
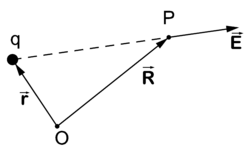
[[Image:Coulombs law.png|right|thumb|250px|Coulomb's law. Electric field '''E''' at point ''P'' due to positive charge ''q''. ]] | [[Image:Coulombs law.png|right|thumb|250px|Coulomb's law. Electric field '''E''' at point ''P'' due to positive charge ''q''. ]] | ||
The force <math>\scriptstyle \vec{\mathbf{F}}</math> divided by Δ''q'' on a test particle of charge Δ''q'' is the [[electrostatic field]] <math>\scriptstyle \vec{\mathbf{E}}</math>. The force originates from one or more charges acting on the test particle. The charge Δ''q'' of the test particle is taken infinitesimally small, that is, it is negligible with respect to the charges causing the field and hence the test particle does not influence the electric field. | The force <math>\scriptstyle \vec{\mathbf{F}}</math> divided by Δ''q'' on a test particle of charge Δ''q'' is the [[electrostatic field]] <math>\scriptstyle \vec{\mathbf{E}}</math>—a vector field. The force originates from one or more charges acting on the test particle. The charge Δ''q'' of the test particle is taken infinitesimally small, that is, it is negligible with respect to the charges causing the field and hence the test particle does not influence the electric field. | ||
The direction of the electric field is by convention such that it points away from a positive charge and points toward a negative charge. | The direction of the electric field is by convention such that it points away from a positive charge and points toward a negative charge. | ||
| Line 37: | Line 37: | ||
</math> | </math> | ||
depending on <math>\scriptstyle \vec{\mathbf{R}}</math>. The sum stands for a vector sum. | depending on <math>\scriptstyle \vec{\mathbf{R}}</math>. The sum stands for a vector sum. | ||
==Coulomb potential== | |||
:''From here on we denote vectors by bold letters dropping the arrows on top of the symbols.'' | |||
When the electrostatic field is irrotational, i.e., | |||
:<math> | |||
\boldsymbol{\nabla} \times \mathbf{E} = \mathbf{0} | |||
</math> | |||
one can define a potential Φ('''r'''), such that, | |||
:<math> | |||
\mathbf{E}(\mathbf{R}) = - \boldsymbol{\nabla} \Phi(\mathbf{R}). | |||
</math> | |||
Since | |||
:<math> | |||
\frac{\partial }{\partial Y} \frac{Z}{R^3} = \frac{\partial }{\partial Z}\frac{Y}{R^3},\quad | |||
\frac{\partial }{\partial X}\frac{Z}{R^3} = \frac{\partial }{\partial Z}\frac{X}{R^3},\quad | |||
\frac{\partial }{\partial Y}\frac{X}{R^3} = \frac{\partial }{\partial X}\frac{Y}{R^3}, | |||
</math> | |||
it follows that '''R'''/''R''<sup>3</sup> is irrotational, and so is ('''R'''-'''r''')/|'''R'''-'''r'''|<sup>3</sup>. The ''Coulomb potential'' Φ exists. The following expression for Φ is consistent with '''E''', | |||
:<math> | |||
\Phi(\mathbf{R}) = \frac{q}{4\pi \epsilon_0 \epsilon_r |\mathbf{R}-\mathbf{r}|} | |||
\quad\Longrightarrow\quad | |||
\mathbf{E}(\mathbf{R}) = - \boldsymbol{\nabla} \frac{q}{4\pi \epsilon_0 \epsilon_r |\mathbf{R}-\mathbf{r}|} | |||
= \frac{q(\mathbf{R}-\mathbf{r})}{4\pi \epsilon_0 \epsilon_r |\mathbf{R}-\mathbf{r}|^3}. | |||
</math> | |||
The Coulomb potential is determined up to a constant. By requiring that Φ vanishes for infinite ''R'' the constant becomes zero. | |||
The Coulomb potential at the point '''R''' due to ''N'' charges at '''r'''<sub>''k''</sub> is | |||
:<math> | |||
\Phi(\mathbf{R}) = \sum_{k=1}^N \frac{q_k}{4\pi \epsilon_0\epsilon_r|\mathbf{R} - \mathbf{r}_k|}. | |||
</math> | |||
For a continuous charge distribution ρ('''r''') this expression generalizes to | |||
:<math> | |||
\Phi(\mathbf{R}) = \iiint \frac{\rho(\mathbf{r})}{4\pi \epsilon_0\epsilon_r|\mathbf{R} - \mathbf{r}|} d\mathbf{r}. | |||
</math> | |||
This generalization follows easily if we first substitute the infinitesimal charge at position '''r''', | |||
:<math> | |||
\Delta q(\mathbf{r}) = \rho(\mathbf{r}) d\mathbf{r} \equiv \rho(\mathbf{r}) dx dy dz , | |||
</math> | |||
and then replace the sum by a volume integral. | |||
==Poisson equation== | |||
==Derivation from Maxwell's equation== | ==Derivation from Maxwell's equation== | ||
Revision as of 08:35, 4 February 2008
In physics, Coulomb's law describes the forces acting between electric point charges. The law was first given by Charles-Augustin de Coulomb. It is an inverse-square law for two electric charges very similar to Newton's gravitational law for two masses. An important difference between the two laws is that masses always attract each other, whereas charges may repel or attract. That is, charges may be positive or negative, while masses have the same sign (are always positive by convention).
Formulation
Coulomb discovered experimentally that the force between two small charged bodies separated in air a distance large compared to their dimensions
- varies directly as the magnitude of each charge,
- varies inversely as the square of the distance between them,
- is directed along the line joining the charges,
- is attractive if the bodies are oppositely charged and repulsive if the bodies have the same type of charge.
Further it was shown experimentally that the force is additive, i.e., the force on a test body exerted by a number of charges around it, is the vector sum of the individual two-body forces. Given two charges q and q' a distance r apart, the force F between the particles is,
The quantities ε0 and εr are the vacuum permittivity and the relative static permittivity (also known as relative dielectric constant) of the medium, respectively. The relative dielectric constant of air is: εr = 1.000536 (at ambient temperature and pressure). The formulation of Coulomb's law is in the rationalized SI system of units.
Electrostatic vector field
The force divided by Δq on a test particle of charge Δq is the electrostatic field —a vector field. The force originates from one or more charges acting on the test particle. The charge Δq of the test particle is taken infinitesimally small, that is, it is negligible with respect to the charges causing the field and hence the test particle does not influence the electric field. The direction of the electric field is by convention such that it points away from a positive charge and points toward a negative charge.
Coulomb's law gives the electric field at the point P due to an electrostatic point charge q at position . The strength of the electric field depends on the inverse-squared distance of P to q and is along the line from q to P, that is,
Note that although this may look like an inverse-cubed dependence, we must not forget that the denominator has dimension length. Indeed, defining a (dimensionless) unit vector by
we see more clearly the inverse-squared dependence on distance:
If q is positive the electric field points from q to P, if q is negative it points from P to q. The force on a particle of charge −|Q| is equal to , that is, the force is antiparallel to the vector . The force on a particle with charge |Q| is equal to , parallel to the vector . In total, charges of opposite sign attract and of same sign repel.
The experimentally observed additivity of electrostatic forces gives the following electric field at P when there are N charges qk at fixed positions rk. The electric field is a vector field
depending on . The sum stands for a vector sum.
Coulomb potential
- From here on we denote vectors by bold letters dropping the arrows on top of the symbols.
When the electrostatic field is irrotational, i.e.,
one can define a potential Φ(r), such that,
Since
it follows that R/R3 is irrotational, and so is (R-r)/|R-r|3. The Coulomb potential Φ exists. The following expression for Φ is consistent with E,
The Coulomb potential is determined up to a constant. By requiring that Φ vanishes for infinite R the constant becomes zero.
The Coulomb potential at the point R due to N charges at rk is
For a continuous charge distribution ρ(r) this expression generalizes to
This generalization follows easily if we first substitute the infinitesimal charge at position r,
and then replace the sum by a volume integral.
Poisson equation
Derivation from Maxwell's equation
(To be continued)
Reference
J. D. Jackson, Classical Electrodynamics, 2nd edition, John Wiley, New York (1975).