Bariatric surgery
This page was started in the framework of an Eduzendium course and needs to be assessed for quality. If this is done, this {{EZnotice}} can be removed.
Obesity is becoming a global pandemic whose manifestations are causing drastic consequences to our health and economy. Currenty Bariatric surgery is the most effective treatment for obesity. In this article we shall discuss the specific bariatric operations and report how surgery effects hormonal modulation of both the gut-brain and adipose-brain axes as opposed to dieting. Our further understading of how bariatric surgery works will yield novel pharmaceuticals in the treamtent for metabolic disorders.
Introduction
Surgery as a treatment for obesity
For many severely obese patients, diets and lifestlye changes do not work in long term. Bariatric surgery has proved to be the only effective method in the long term treatment of obesity.
How surgery works
Bariatric surgery works by altering the anatomy of the gastrointestinal tract, thus: limiting the volume of food the stomach can hold, interfering with the absorption of calories, and by interfering with orexigenic and anorexigenic signalling between the gut, adipose tissues and the brain.
Bariatric Surgical Procedures
Restrictive Surgery
Restrictive surgery reduces the size of the stomach causing decreased hunger and an increased satiety following ingestion of smaller amounts of food. Surgical procedures include gastric banding, where an adjustable band is clipped just below the cardia of the stomach creating a small gastric pouch above(kerrigan, le roux). Other forms of restrictive surgery includes gastroplasty and sleeve gasrectomy.
Malabsorptive Surgery
Jejuno-ileal bypass, duodenojejunal bypass and biolopancreatic bypass (with or without duodenal switch)
Combination Surgery
Combination surgery combines the benefits of both restrictive and restrictive surgery. The most common bariatric procedure in the US is the Roux-en-Y Gastric Bypass (RYGP).
Duodenal switch
Complications and Side effects
Surgical Effects Regarding Gut Hormones
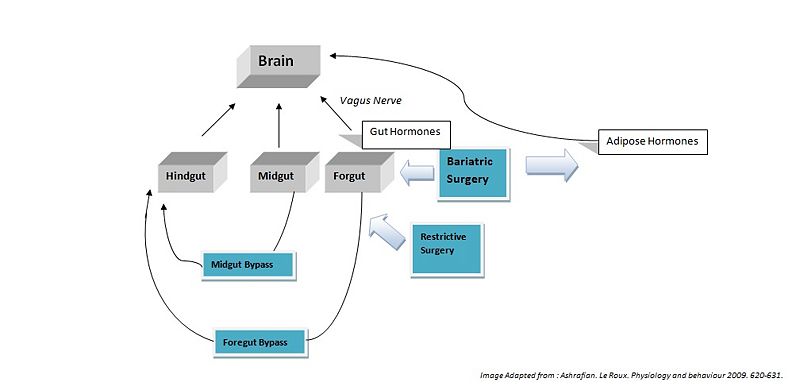
Hindgut Hormones
Peptide tyrosine tyrosine (PYY) is a peptide belonging to the neuropeptide Y (NPY) family [1]. PYY is normally released in proportion to calorie intake and has recently been described as an important feedback molecule in the Gut-Brain axis. As well as being a satiety signal PPY has also been suggested to increase energy expenditure [2]. Following bypass and restrictive surgery, basal and postprandial PYY levels have been shown to increase [3]. In addition basal PYY levels have been shown to increase following illial resection, and patients have been reported to have lost weight (broberger,2005). By contrast, it was found that diet-induced weight loss did not increase PYY levels (Olivan, 2009).
Glucagon-like peptide-1 (GLP-1), acts similarly to PYY, acting in the hypothalamus as an anorexigenic signal. The literature shows the majority of patients following foregut bypass surgery show a marked increase in basal and postprandial GLP-1. Midgut bypass also shows an increase in GLP-1 however studies on restrictive surgery have shown either no change or a decrease in GLP-1 basal levels. Of specific interest GLP-1 has been shown to improve postpriandal glycaemic control, being a potent incretin mimetic. A GLP-1 agonist, exenatide has not yet been accepted for use as an anti-obesity drug; however it is already being used in the treatment of type 2 diabetes mellitus (Ashrafian, 2009).
Midgut Hormones
Neurotensin (NT), is a well know anoregigenic signal where its expression has been shown to be downregulated in ob/ob leptin defficient mice. Following bypass surgerys (5 of 6 reported) an increase in NT levels has been demonstrated which could contribute to the anorexigenic effects of the bypass operations (Ashrafian, 2009).
Foregut Hormones
Ghrelin is an orexigenic hormone and is released from the fundus of stomach. It is released into the blood to promote eating, and levels begin to fall after meals when satiety is reached. Ghrelin is also believed to play a role in regulating body weight over a long period of time. As a result, ghrelin levels increase in order to compensate for the starvation induced by reduced calorie intake observed in diets. It would be assumed that bariatric surgery reduces hunger by lowering the amount of ghrelin released however the results from the literature remain controversial. Of a total of 42 studies following forgut bypass surgerys, only 50% reported a decrease in ghrenlin release. A large number of these studies reported ghrelin levels immediately after surgery, whereas studies of long term ghrelin change following surgery show no significant trend. Results for restrictive surgeries shows only a 18% decrease in ghrenlin levels. These results indicated bariatric surgeries may not directly modulate Grenlin release (Korner et al., 2005 and Holdstock et al., 2003).
Cholecystokinin (CKK) was the first anoregixigenic gut peptide to be implicated in the control of appetite (murphy). One would speculate that CCK would rise following bariatric surgery however the results from the literature have been disappointing and suggest no role in the mediation of CCK following bariatric surgery (ashrafan).
Gastrin
Glucose-dependent Insulinotrophic Peptide (GIP) is a hormone produced in the foregut. It is released when simple, energy-dense nutrients like glucose and free fatty acids are present and it promotes nutrient (energy) storage (Rosen, 2009). Those suffering with obesity and diabetes often have a diet-induced, chronic elevation in GIP levels with peripheral tissue receptor down-regulation that is analogous to insulin insensitivity. Therefore, it would be optimal to decrease GIP levels and improve GIP sensitivity to lose weight. It has been showed that diet-induced weight loss increases GIP levels after a nutrient-rich meal and prevents further weight loss because the body counters the perceived starvation by increasing nutrient deposition in central and peripheral stores. By contrast, proximal fogut bypass surgery, roux-en-Y gastric bypass and biliopancreatic diversion with duodenal switch were found to decrease GIP levels and hence promote weight loss.
Other Hormones
Surgical Treatment as Opposed to Diet
It should be noted that this area of how bariatric surgery works is still quite controversial. Nevertheless, clinical observations demonstrate the effectiveness of surgery as opposed to diet. An attractive theory is as follows; when an individual consumes excessive amounts of calories satiety signals become abundant and their corresponding receptors become either desensitized or down regulated. Now when this obese individual attempts to lose weight by diet, satiety signals are ineffective. Thus the individual remains hungry. In addition in obese individuals following diets, body metabolism is so low that it makes dieting near to impossible. Bariatric surgery is proposed to work by increasing energy metabolism, reducing orexigenic signals, increasing anorexigenic signals and interfering with calorie absorption. For instance, after diet induced weight loss orexigenic hormones like ghrelin are relesased in abundance as the body is in 'starvation mode' - increasing the chances of the individual to over-eat to regain energy therefore regaining the weight. However, even though bariatric surgery similarly induces weight loss - the reduction in stomach size allows a proportionate decrease in ghrelin released. Hence, even though there is reduced calorie intake - the body does not try to compensate as less orexigenic signals are released. The precise functioning of these systems shall remain at the heart of controversy. Physiological reasoning is beyond the scope of this article however must also be considered (Korner et al., 2005).
Normally, obese people tend to have lower PYY levels compared to those who are lean, under fasting conditions. A year after bariatric surgery, such as jejunoileal bypass and vertical banding, the levels of fasting PYY was found to have increased. Moreover, concentrations within an hour of eating did not seem to change considerably - whether in an obese, lean, or postoperative. However, after Roux-en-Y gastric bypass, it has been reported that there is a postprandial PYY surge. There are no clear indications as to what might lead to this, however it is strongly believed that the reason for this exaggerated release is the removal of the pylorus (as well as a portion of the stomach) that allows food to enter the small intenstine at a faster rate. This early rise in PYY levels will therefore also induce satiety early and reduce food intake. Again, even though such cases have in fact been reported - controversy still surrounds the exact mechanisms and affects surgery has on PYY levels due to the variability between individuals. There has not been enough postprandial PYY results from larger number of individuals that with strong statistical indications can correlate significant changes to satiety induction as a result of PYY level alterations post-operatively (Korner et al., 2005).
Type II Diabetes is usually developed in approximately 41% of obese cases as the body becomes resistant to insulin and inadequately responds to glucose [2]. Diet induced weight loss certainly ameliorates the insulin response but not to the extent where euglycemia is restored and insulin is normally released in response to glucose levels. Furthermore, the usual cases where weight is re-gained after diets will almost certainly lead to the re-establishment of insulin resistance and inadequate insulin response to glucose. After biliopancreatic diversion with Roux-en-Y gastric bypass, patients insulin sensitivity had improved greatly, and its release according to glucose levels was also improved. It is important to take note that, even within a year of bariatric surgery patients are usually still obese (with a BMI of ~ 30 kg/m2) however after massive weight loss. Yet, the improvements demonstrated of insulin resistance and its response to glucose had taken place a few months after the operation. Therefore, the initial improvement of type 2 diabetes seems to take place even as the patient is still obese. This is a great asset to surgical weight loss as opposed to diet induced weight loss (Polyzogopoulou et al., 2003).
Future Possibilites and Conclusion
There is no doubt that further understanding of the physiology of how each specific operation effects hormonal modulation will lead to new pharmaceutical treatments in the treatment of obesity and appetite disorders.
Conclusion
References
Broberger, C. "Brain Regulation of food intake and appetite: molecules and networks" Journal of internal Medicine (2005); 258: 301-327.[3]
DeMaria "Bariatric Surgery for Morbid Obesity". The new England Journal of Medicine (2007); 356; 2176-83 [4]
Murphy, K.G. "Gut Hormones in the control of appetite" Exp Physiol 89. pp 507-616. [5]


