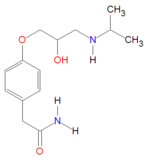
Atenolol
|
| |||||||
| atenolol | |||||||
| |||||||
| Uses: | hypertention;angina pectoris | ||||||
| Properties: | hydrophilic | ||||||
| Hazards: | see side effects & drug interactions | ||||||
| |||||||
In medicine, atenolol is a cardioselective adrenergic beta-antagonist that is "possessing properties and potency similar to propranolol, but without a negative inotropic effect."[1] Atenolol is hydrophilic[2]
History
Atenolol was developed by the Stuart Company which was a division of Imperial Chemical Industries (ICI). ICI was renamed Zeneca in 1992. Atenolol received approval in the United States August 19, 1981.[3] According to drugstore.com, 90 days of generic 50 mg pills costs $17.99 in January, 2009.
Metabolism
Atenolol is excreted unchanged in the kidneys. Elimination is dependent on the glomerular filtration rate. Atenolol is not metabolized in the liver by cytochrome P-450 2D6 allele.
Dosage
For healthy adults, the starting dose recommended by the manufacturer is 50 mg orally once daily and the maximum dose is 100 mg orally once daily. However, atenolol may require twice daily dosing[4]
Chemistry
Atenolol may defined by IUPAC nominclature as 4-[2'-hydroxy-3'-[(1-methylethyl)amino]propoxy-benzeneacetamide. It is a hydrophilic drug, with solubility in water equal to 26.5 mg/mL at 37ºC, with chemical formula C14H22N2O3 andmolecular mass 266.34 gram/mole for the free base form. It is freely soluble in strongly acidic solutions.
Efficacy
Coronary heart disease
Heart failure
Although atenolol has not received indication in the United States for the treatment of heart failure, two cohort studies suggest that the beta-blockers atenolol and carvedilol may be more effect than metoprolol for the treatment of heart failure.[5][6]
Randomized controlled trials by one research group also suggest atenolol might benefit.[7][8]
Hypertension
External links
The most up-to-date information about Atenolol and other drugs can be found at the following sites.
- Atenolol - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Atenolol - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Atenolol - Detailed information from DrugBank.
References
- ↑ Anonymous (2024), Atenolol (English). Medical Subject Headings. U.S. National Library of Medicine.
- ↑ Tuininga YS, Crijns HJ, Brouwer J, et al (December 1995). "Evaluation of importance of central effects of atenolol and metoprolol measured by heart rate variability during mental performance tasks, physical exercise, and daily life in stable postinfarct patients". Circulation 92 (12): 3415–23. PMID 8521562. [e]
- ↑ Drugs@FDA. U S Food and Drug Administration
- ↑ Sarafidis P, Bogojevic Z, Basta E, Kirstner E, Bakris GL (February 2008). "Comparative efficacy of two different beta-blockers on 24-hour blood pressure control". J Clin Hypertens (Greenwich) 10 (2): 112–8. PMID 18259123. [e]
- ↑ Kramer JM, Curtis LH, Dupree CS, et al (December 2008). "Comparative effectiveness of beta-blockers in elderly patients with heart failure". Arch. Intern. Med. 168 (22): 2422–8; discussion 2428–32. DOI:10.1001/archinternmed.2008.511. PMID 19064824. Research Blogging.
- ↑ Go AS, Yang J, Gurwitz JH, Hsu J, Lane K, Platt R (December 2008). "Comparative effectiveness of different beta-adrenergic antagonists on mortality among adults with heart failure in clinical practice". Arch. Intern. Med. 168 (22): 2415–21. DOI:10.1001/archinternmed.2008.506. PMID 19064823. Research Blogging.
- ↑ Sturm B, Pacher R, Strametz-Juranek J, Berger R, Frey B, Stanek B (December 2000). "Effect of beta 1 blockade with atenolol on progression of heart failure in patients pretreated with high-dose enalapril". Eur. J. Heart Fail. 2 (4): 407–12. PMID 11113718. [e]
- ↑ Hülsmann M, Sturm B, Pacher R, et al (November 2001). "Long-term effect of atenolol on ejection fraction, symptoms, and exercise variables in patients with advanced left ventricular dysfunction". J. Heart Lung Transplant. 20 (11): 1174–80. PMID 11704477. [e]