Ammonia production
Ammonia production facilities provide the base ammonia used predominantly in fertilizers supplying usable nitrogen for agricultural productivity. Ammonia is one of the most abundantly-produced inorganic chemicals. There are literally dozens of large-scale ammonia production plants throughout the industrial world, some of which produce as much as 2,000 to 3,000 tons per day of ammonia in liquid form. The worldwide production in 2006 was 122,000,000 metric tons.[1] China produced 32.0% of the worldwide production followed by India with 8.9%, Russia with 8.2%, and the United States with 6.5%. Without such massive production, our agriculturally-dependent civilization would face serious challenges.
Why do we need to manufacture ammonia on an industrial scale
....
History of ammonia manufacturing processes
Before the start of World War I, most ammonia was obtained by the dry distillation of nitrogenous vegetable and animal products; the reduction of nitrous acid and nitrites with hydrogen; and the decomposition of ammonium salts by alkaline hydroxides or by quicklime, the salt most generally used being the chloride (sal-ammoniac).
The Haber process, which is the production of ammonia by combining hydrogen and nitrogen, was first patented by Fritz Haber in 1908. In 1910, Carl Bosch, while working for the German chemical company BASF, successfully commercialized the process and secured further patents. It was first used on an industrial scale by the Germans during World War I. Since then, the process has often been referred to as the Haber-Bosch process.
Modern ammonia-producing plants
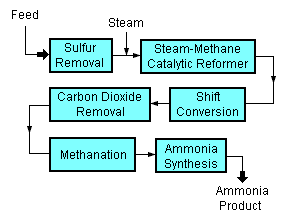
A typical modern ammonia-producing plant first converts natural gas (i.e., methane) or LPG (liquified petroleum gases such as propane and butane) or petroleum naphtha into gaseous hydrogen. The method for producing hydrogen from hydrocarbons is referred to as "steam reforming".[2][3][4] The hydrogen is then combined with nitrogen to produce ammonia. [5][6]
Starting with a natural gas feedstock, the processes used in producing the hydrogen are:
- The first step in the process is to remove sulfur compounds from the feedstock because sulfur deactivates the catalysts used in subsequent steps. Sulfur removal requires catalytic hydrogenation to convert organic sulfur compounds (RSH) in the feedstocks to gaseous hydrogen sulfide (H2S):
- H2 + RSH → RH + H2S(gas)
- The gaseous hydrogen sulfide is then absorbed and removed by passing it through beds of zinc oxide (ZnO) where it is converted to solid zinc sulfide (ZnS):
- H2S + ZnO → ZnS + H2O
- Catalytic steam reforming of the sulfur-free methane (CH4) feedstock is then used to form carbon monoxide (CO) plus hydrogen (H2): :
- CH4 + H2O → CO + 3H2
- The next step then uses catalytic shift conversion to convert the carbon monoxide to carbon dioxide (CO2) and more hydrogen:
- CO + H2O → CO2 + H2
- The carbon dioxide is then removed either by absorption in aqueous ethanolamine solutions or by adsorption in pressure swing adsorbers (PSA) using proprietary solid adsorption media.
- The final step in producing the hydrogen is to use catalytic methanation to remove any small residual amounts of carbon monoxide or carbon dioxide from the hydrogen by converting them into methane:
- CO + 3H2 → CH4 + H2O
- CO2 + 4H2 → CH4 +2H2O
To produce the desired end-product ammonia, the hydrogen is then catalytically reacted with nitrogen (N2) derived from process air to form anhydrous liquid ammonia (NH3). This step is known as the "ammonia synthesis loop" (also referred to as the Haber-Bosch process):
- 3H2 + N2 → 2NH3
The steam reforming, shift conversion, carbon dioxide removal and methanation steps each operate at absolute pressures of about 25 to 35 bar, and the ammonia synthesis loop operates at absolute pressures ranging from 60 to 180 bar depending upon which proprietary design is used. There are many engineering and construction companies that offer proprietary designs for ammonia synthesis plants. Haldor Topsoe of Denmark, Technip of France, Uhde GmbH of Germany, and Kellogg Brown & Root of the United States are among the most experienced companies in that field.
Uses of ammonia
About 80% or more of the ammonia produced is used for fertilizing agricultural crops in the form of aqua ammonia (an aqueous solution of ammonia), ammonium sulfate (NH4)2SO4, ammonium phosphate (NH4)3PO4, ammonium nitrate NH4NO3 and urea (NH2)2CO.
Ammonia is also used for:
- Manufacture of nitric acid (HNO3)
- Manufacture of nylon and other polyamides
- Refrigerant in household, commercial and industrial refrigeration systems
- Manufacture of dyes
- Manufacture of explosives
- Cleaning solutions
References
- ↑ United States Geological Survey publication
- ↑ Gary Maxwell (2004). Synthetic Nitrogen Products: A Practical Guide to the Products and Processes, First Edition. Springer. ISBN 0-306-48225-8.
- ↑ Twygg, Martyn V. (1989). Catalyst Handbook, 2nd Edition. Oxford University Press. ISBN 1-874545-36-7.
- ↑ Samuel Strelzoff (1987). Technology and Manufacture of Ammonia. Krieger Publishing. ISBN 0-89464-250-2.
- ↑ J.R. Jennings (Editor) (1991). Catalytic Ammonia Synthesis, First Edition. Springer. ISBN 0-306-43628-0.
- ↑ Sami Matar and Lewis F. Hatch (2001). Chemistry of Petrochemical Processes, Second Edition. Gulf Publishing. ISBN 0-8841-5315-0.
- Pages using ISBN magic links
- Editable Main Articles with Citable Versions
- CZ Live
- Engineering Workgroup
- Chemistry Workgroup
- Chemical Engineering Subgroup
- Articles written in American English
- Advanced Articles written in American English
- All Content
- Engineering Content
- Chemistry Content
- Chemical Engineering tag