Staphylococcus aureus
| Staphylococcus aureus | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| File:Staphylococcus-aureus.jpg | ||||||||||||
| Scientific classification | ||||||||||||
| ||||||||||||
| Species | ||||||||||||
|
Staphylococcus aureus |
Staphylococcus aureus is a Gram-positive spherical coccus that grows in a loose, irregular cluster resembling clusters of grapes. The cluster formation is due to the cell division occurring in three planes, with the daughter cell remaining in nearby. [1] Staphylococcus aureus may also be found singly, in pairs and in short chains of three or four cells. The bacterium can never be found in long chains. They are non motile and non spore forming. On an ordinary medium, Staphylococcus aureus can grow within a temperature range of 10-42°C. The optimum pH ranges in between pH 7.4-7.6. The bacterium thrives best in an oxygen rich environment. S. aureus can grow on all common laboratory media such as milk, nutrient gelatin or agar. When grown on an agar-based culture medium and incubated for 24 hours the colonies appear to be 2-4mm in diameter.[2] The colonies appear circular, smooth, convex, shiny, and opaque.[3]
Staphylococcus aureus was first observed in 1871 by von Recklinghausen but not isolated. Then in 1881 a surgeon by the name of Alexander Ogston documented two kinds of micrococci. The already known streptococci, arranged in chains and the other cocci arranged in clusters. Ogston named the cocci cluster Staphylococci because “Staphyle” in Greek means "bunches of grapes" and “kokakos” meaning a "berry".[4] Unfortunately, Ogston did not provide a description of the genus and therefore it was not recognized. In 1884 Rosenbach successfully isolated and grew Staphylococcus aureus from pus. Rosenbach is credited with proposing the genus Staphylococcus and the species Staphylococcus aureus.[5] He kept the genus name Staphlococcus because the bacteria was similar to that studied by Ogston. Rosenbach proposed the nomenclature for Staphylococcus aureus based on the yellow pigmentation of the colony.
In recent years Staphylococcus aureus has become a serious health issue. The bacteria has built up a strong resistance to treatment. By unlocking the genome sequence, researchers could understand the nature of its resistance, virulence, genetic flexibility, epidemiology and physiology. Comparing the genome sequence of S. aureus with the genomes of a less virulent form and nonpathogenic species could help researchers have a better understanding of the nature of Staphylococcal aureus infections.
Genome structure
The Staphylococcus aureus genome contains about 2.800 to 2.903 million base pairs of DNA. The bacteria has about 2,600 genes in its chromosome. The first whole genome sequence of S. aureus strains were completed by random shot-gun sequencing in 2001. S. aureus plasmids contains genes that encode resistance to antibiotics, heavy metals, or bacteriocides.[6] Some virulence genes have been reported to be carried on plasmid, such as exfoliative toxin B and some superantigens.[7] Approximately 75% of S. aureus genome comprises a core component of genes present in all of the strains.[8]
Cell structure and metabolism
Staphylococcus aureus cell wall contains a thick peptidoglycan layer and teichoic acid. The polysaccharide peptidoglycan in the cell wall gives the bacterium structure and rigidity and induces the release of cytokines. Teichoic acid is an antigenic component that aids adhesion of the cocci to the host cell surface. The bacterium contains no flagella. Some young cultures posses microscopically visible capsules. Many noncapsulated strains of S.aureus have small amounts of capsular material on the surface.[9]
Staphylococci are facultative anaerobes that grow by aerobic respiration or by fermentation that yields lactic acid. S. aureus ferments sugars by producing acid but no gas.
Phagocytosis is a mechanism used by the host organism to combat a staphylococcal infections. S. aureus produces leukocidin, which cause the destruction of leukocytes allowing the bacteria to escape phagocytosis.[10] Leukocidin is produced in skin lesions such as boils which results in cell destruction of white blood cells and is one of the factors responsible for pus formation.
Ecology
Staphylococcus aureus is commonly found on the skin and in various mucous membranes of man and other animals. About 20-30% of healthy people in the United States are carriers of the bacteria.[11] These individuals are usually unaware that they are carriers of the bacteria and usually never get sick from it. In hospitals the percentage is higher because of more possible contact with infected cases.
Pathology
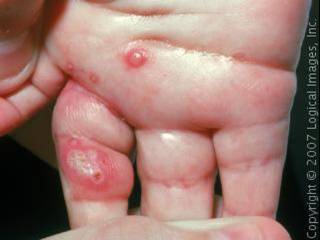 Staphyolococcus aureus may cause furuncles (boils).
Staphyolococcus aureus may cause furuncles (boils).
File:Staphycoccal scalded skin syndrome(SSSS). .jpg The exfoliative (epidermolytic)toxin is responsible for staphycoccal scalded skin syndrome(SSSS).
Staphylococcus aureus is one of the most common pathogenic bacteria. The organism may cause disease through tissue invasion and toxin production. The bacteria could cause a wide range of infections both internally and externally. It may cause skin infections, bone infections, pneumonia, food poisoning, Toxic shock syndrome, bacterial endocarditis, life threatening bloodstream infections and other serious illnesses.
Staphyolococcus aureus may cause skin abscesses (boils) usually by entering the skin through a hair follicle or a cut. They may be red, swollen and painful, and sometimes have pus. They can turn into impetigo, which turns into a crust on the skin and usually common among newborns. The organism could also cause internal abscesses.
In recent years Staphylococcus aureus has become one of the leading causes of cross infections (hospital acquired infections). People prone to staphylococcal infections include newborns, drug users, breastfeeding women, and people with skin disorders, surgical incisions, a weakened immune system, or chronic diseases.[12]
Extracellular and cell associated factors may influence virulence. Cell surface proteins, extracellular enzymes and toxins are present on most strains of S. aureus and increases the species ability to act as a successful pathogen.
Protein A and clumping factors are both cell surface proteins. Protein A induces platelet damage and hypersensitivity. The cocci clumps when introduced to human plasma because of this researchers use a coagulase test to help identify the bacterium. Some strains may not always test positive because they may be encapsulated.
Coagulase, nucleases, lipases, hyaluronidase and protein receptors are all extracellular enzymes that play an important role in pathogenesis. The bacteria can convert fibrinogen to fibrin, has a heat stable nuclease, produces lipid hydrolases which aids in infecting the skin, breaks down connective tissue, and possess receptors that facilitate adhesion to the host cell and tissue.
Toxins such as alpha hemolysin, enterotoxin, toxic shock syndrome toxin(TSST), and exfoliative (epidermolytic)toxin produced by S. aureus may cause the bacerium to be more virulent. Alpha hemolysin is a protein that is inactivated at 70oC but activated at 100oC. It is toxic to macrophages, lysosomes, muscle tissues, renal cortex, and the circulatory system. Enterotoxin is also a superantigen responsible for causing food poisoning which may lead to nausea, vomiting, and diarrhea. Toxic shock syndrome toxin is a super antigen as well and causes toxic shock syndrome in the infected host. It may prove to be a potentially fatal multi system disease. The infected host can experiance a fever, hypotension, myalgia, vomiting, diarrhea and mucosal hyperemia. The exfoliative (epidermolytic)toxin is responsible for staphylococcal scalded skin syndrome(SSSS). It is an exfoliative skin disease which causes the outer layer of the epidermis to be separated from the underlying tissues. Symptoms associated with the disease are a fever, malaise and irritability following an upper respiratory infection.
Staphylococcus aureus has a high incidence of drug resistance with methicillin-resistant strains resistant to ß-lactams and most other antibiotics. Infections are enhanced in the presence of foreign materials inside the body such as tampons, surgical packing or intravenous catheters.
Application to Biotechnology
In an anaerobic environment Staphylococcus aureus can reduce mannitol to lactic acid, which differentiates it from other species of staphylococci. The bacterium is the only coagulase positive and ß-hemolytic staphylococcus. When grown on a nutrient agar containing phenolphthalein diphosphate the bacterium produces phosphatase. When ammonia vapor is introduced to the culture, the colonies assume a bright pink color due to the presence of free phenolphthalein. Researchers use this test to distinguish between Staphylococcus aureus from S. epidermidis.
S. aureus is coagulase positive, ferments mannite, produce clear hemolysis on blood agar, liquefy gelatin, produces phosphatase, in a medium containig potassium it reduce tellurite to form black colonies and it produce thermostable nucleases.
Current Research
- See also: Cross infection
There has always been a need for universal screening at hospitals for methicillin-resistant Staphylococcus aureus (MRSA) at time of admission of patients.[13] A study was conducted from July 2004 to May 2006 to demonstrate the effect of early detection of MRSA infection rates in surgical patients. There were about 21, 754 patients involved in this study. The study compared rapid screening on admission plus standard infection control measure vs. standard infection control alone. The study showed that 94% of patients were screened at time of admission to the hospital. The screening identified 5.1% of the patients were MRSA positive and more that half of the 5.1% was unaware that they were carriers. During the rapid screening period 93 patients developed nosocomial MRSA infections and only 73 patients during the control period. The study reveled that universal screening for methicillin-resistant Staphylococcus aureus did not reduce nosocomial MRSA infections in surgical patients. [14]
The study examined Staphylococcus aureus strains from skin lesions for their potential to produce immune system- modulating toxins and to connect it to the number of white blood cells found in the infected skin lesions. There were 84 isolated bacterial chromosomal DNA obtained. They were categorized into two groups, those that correlated with a low white blood cell count and those with a high white blood cell count. The study indicated that there were a higher number of bacteria capable of producing exfoliative toxins A or B and Panton-Valentine leukocidin without taking into account the number of white blood cells. The research revealed that the S. aureus associated with a low number of white blood cells produced exfoliative toxins A or B. S. aureus associated with a high number of white blood cells produced toxins such as Panton-Valentine leukocidin and toxic shock syndrome toxin. The infected skin lesions appeared no different from each other.
George Liu, Anthony Essex, John Buchanan, Vivekanand Datta, Hal Hoffman, John Bastian, Joshua Fierer and Victor Nizet.(2005) Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. The Journal of Experimental Medicine, 202. Retrieved April 12, 2008 from [The Journal of Experimental Medicine]
The research conducted demonstrated that the golden pigmentation of Staphylococcus aureus is a virulence factor and could potentially to lead antimicrobial therapy. An S. aureus mutant was created with a disrupted carotenoid biosynthesis pathway. The pigment proved to have antioxidant properties. The study showed that a mutant strain missing the carotenoid coating was less pathogenic making it more vulnerable to free radicls. When the carotenoid biosynthesis pathway is inhibited there is an increase oxidant sensitivity. The S. aureus carotenoid play an important role in its resistance to neutrophil mediated killing. The inhibition of the carotenoid biosynthesis pathway may lead to a therapeutic approach to the treatment of Staphylococcus aureus infections.
Methicillin-resistant staphylococcus aureus
Methicillin-resistant staphylococcus aureus (MRSA) is a strain of Staphylococcus aureus that is resistant to commonly used antibiotics such as methicillin. MRSA emerged in the early 1960's. MRSA is predominantly a nosocomial pathogen causing hospital acquired infections as well as community acquired infections. Currently available statistics from the Kaiser foundation in 2007 indicate that about 1.2 million hospitalized patients have MRSA, and the mortality rate is estimated to be between 4%-10%.[15]
MRSA may be more virulent than other staphylococcus aureus due to carrying the gene for Panton-Valentine leucocidin (PVL). [16]
Screening for MRSA
In order to prevent the spread of the bacterium in hospitals, it would seem that patients who are infected need to be identified as soon as possible. Many antibiotics are ineffective for treating sever infections and early identification of patients is an essential precautions to take to prevent the spread.
Regarding the impact of screening:
- A randomized controlled trial reported reduction in surgical infections by about 5%[17]
- A cluster randomized controlled trial reported small benefits from screening in various types of hospital wards.[18] Although the authors of the trial doubted the cost of screening was justified, the authors did not conduct a formal cost analysis. Another randomized controlled trial of screening surgical patients found no benefit.[19]
As of 2008 Illinois, New Jersey, and Pennsylvania have passed laws requiring hospitals to screen certain patients upon admission for MRSA. All three states require hospitals to screen patients admitted to intensive care units and high risk patients in other parts of the hospital to identify those colonized with MRSA.[20]
Diagnosis
Skin abscesses due to MRSA are more likely to have a central black eschar (sensitivity 55%; specificity 92%).[21]
Eradication of MRSA
- See also: Cross infection
It is possible to reduce the risk of MRSA infections and transmission. Washing hands regularly, use an alcohol based hand rub, have good housekeeping skills by using disinfectants such as quaternary ammonium compounds, skin wounds should be covered with dressings to avoid exposure or isolating the infected.
Normally skin infection may not need treatment. While others may require incision and drainage of the infected area and the use of antibiotics.
Methicillin-resistant staphylococcus aureus is resistant to many types of antibiotics but may be treatable. Vancomycin is the most popular antibiotic used to treat infections. MRSA can also be treated teicoplanin or linezolid.[22] If the infection is community acquired MRSA and non-serious, the patient can be treated without a hospital admission using trimethoprim-sulfamethoxazole or clindamycin.
Sometimes a patient has recurrent infections caused by MRSA. Combination therapy is required if decolonization is going be attempted. Decolonization does not always work. The patient is being subjected to more antibiotics which could cause other factors such as elimination of indigenous flora (giving rise to Clostridium difficile pseudomembranous colitis) or development of more resistant organisms.
References
- ↑ Paniker CKJ (2006). Textbook of Microbiology, 7th. Andhra Pradesh, India: Orient Blackswan. ISBN 8125028080.
- ↑ Textbook of Microbiology
- ↑ Textbook of Microbiology
- ↑ Medicinenet: Staphylococcus aureus
- ↑ Textbook of Microbiology
- ↑ Insights on Virulence and Antibiotic Resistance: A Review of the Accessory Genome of Staphylococcus aureus
- ↑ Yamaguchi T, Hayashi T, Takami H, et al. Complete nucleotide sequence of a Staphylococcus aureus exfoliative toxin B plasmid and identification of a novel ADPribosyltransferase, EDIN-C. Infect Immun. 2001;69(12):7760-7771.
- ↑ Lindsay JA, Holden MTG. Understanding the rise of the superbug: investigation of the evolution and genomic variation of Staphylococcus aureus. Funct Integr Genomics. 2006;6(3):186-201
- ↑ Textbook of Microbiology
- ↑ Brock, Madigan, Martinko, Parker. Biology of Microorganisms New Jersey: Prentice Hall, 1994.
- ↑ Medicinenet: Staphylococcus aureus
- ↑ Merck: Staphylococcal Infections
- ↑ Harbarth S, Frankhauser C, Schrenzel J, Christenson J, Gervaz P, Bandiera-Clerc C, Renzi G, Vernaz N, Sax H, Pittet (2008), "Galenicom.com] Universal screening for methicillin-resistant Staphylococcus aureus at hospital admission and nosocomial infection in surgical patients]", JAMA
- ↑ Patricia M. Mertz; Tatiana C. P. Cardenas; Richard V. Snyder; Megan A. Kinney; Stephen C. Davis; Lisa R. W. Plano (2007), "[http://archderm.ama-assn.org/cgi/content/abstract/143/10/1259 Staphylococcus aureus Virulence Factors Associated With Infected Skin Lesions Influence on the Local Immune Response]", Arch Dermatol 143
- ↑ "MRSA Infection", Medicinenet
- ↑ Rajendran PM, Young D, Maurer T, et al (2007). "Randomized, Double-Blind, Placebo-Controlled Trial of Cephalexin for Treatment of Uncomplicated Skin Abscesses in a Population at Risk for Community-Acquired Methicillin-Resistant Staphylococcus aureus Infection". Antimicrob. Agents Chemother. 51 (11): 4044–8. DOI:10.1128/AAC.00377-07. PMID 17846141. Research Blogging.
- ↑ Bode LG, Kluytmans JA, Wertheim HF, Bogaers D, Vandenbroucke-Grauls CM, Roosendaal R et al. (2010). "Preventing surgical-site infections in nasal carriers of Staphylococcus aureus.". N Engl J Med 362 (1): 9-17. DOI:10.1056/NEJMoa0808939. PMID 20054045. Research Blogging.
- ↑ Jeyaratnam D, Whitty CJ, Phillips K, et al (April 2008). "Impact of rapid screening tests on acquisition of meticillin resistant Staphylococcus aureus: cluster randomised crossover trial \". BMJ 336 (7650): 927–30. DOI:10.1136/bmj.39525.579063.BE. PMID 18417521. PMC 2335244. Research Blogging.
- ↑ Harbarth S, Fankhauser C, Schrenzel J, et al (March 2008). "Universal screening for methicillin-resistant Staphylococcus aureus at hospital admission and nosocomial infection in surgical patients". JAMA 299 (10): 1149–57. DOI:10.1001/jama.299.10.1149. PMID 18334690. Research Blogging.
- ↑ Infectioncontroltoday: More States Move to Require Hospitals to Screen Patients for MRSA
- ↑ Busch BA, Ahern MT, Topinka M, Jenkins JJ, Weiser MA (July 2008). "Eschar with cellulitis as a clinical predictor in community-acquired MRSA skin abscess". J Emerg Med. DOI:10.1016/j.jemermed.2007.11.072. PMID 18614328. Research Blogging.
- ↑ Anonymous (2009). Drugs for MRSA with Reduced Susceptibility to Vancomycin.
http://www.nih.org/NIHnewWebsite/nihPublicHealth/pdfs/MRSAParentsGuide.pdf