Cefalotin: Difference between revisions
imported>David E. Volk (New page: {{subpages}} {{Chem infobox |align=right |image=center|thumb|300px|{{#ifexist:Template:Cefalotin.jpg/credit|{{Cefalotin.jpg/credit}}<br/>|}} |width=300px |molname=...) |
imported>David E. Volk m (typo) |
||
| Line 16: | Line 16: | ||
}} | }} | ||
'''Cefalotin''', also called '''sefalothin''', is a semisynthetic first generation [[cephalosporin]] [[antibiotic]] compound that is structurally similar to other cephalosporins including [[cefaclor]], [[ | '''Cefalotin''', also called '''sefalothin''', is a semisynthetic first generation [[cephalosporin]] [[antibiotic]] compound that is structurally similar to other cephalosporins including [[cefaclor]], [[cefadroxil]] and [[cefazolin]]. | ||
It is administered [[parenterally]] to prevent infections during surgery or for treating a broad spectrum of infections of the blood, bone or joints, respiratory tract, skin, and urinary tract. | It is administered [[parenterally]] to prevent infections during surgery or for treating a broad spectrum of infections of the blood, bone or joints, respiratory tract, skin, and urinary tract. | ||
Revision as of 16:08, 24 April 2008
|
| |||||||
| cefalotin | |||||||
| |||||||
| Uses: | antibiotic drug | ||||||
| Properties: | beta-lactam | ||||||
| Hazards: | see drug interactions | ||||||
| |||||||
Cefalotin, also called sefalothin, is a semisynthetic first generation cephalosporin antibiotic compound that is structurally similar to other cephalosporins including cefaclor, cefadroxil and cefazolin. It is administered parenterally to prevent infections during surgery or for treating a broad spectrum of infections of the blood, bone or joints, respiratory tract, skin, and urinary tract.
Chemistry
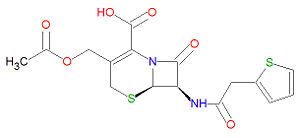
The IUPAC chemical name for cefalotin is (6R,7R)-3-(acetyloxymethyl)-8-oxo-7-[(2-thiophen-2-ylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid and it has chemical formula C16H16N2O6S2, giving it a molecular mass of 396.4380 g/mol. The presence of a beta-lactam moiety makes it susceptible to degradation by [[beta-lactamase]s present in some bacterial strains. The structure of cefalotin and other cephalosporins resemble those of penicillins, but have a six-membered ring attached to the four-membered ring, wherease the penicillins have a five-membered ring attached to the four-membered ring.
Mechanism of action
Like penicillin and other cephalosporins, cefalotin works by disrupting the last stage of bacterial cell wall synthesis, leading to autolysis of the bacteria cells by autolytic enzymes such as the autolysins. Some bacteria gain the ability to produce beta-lactamases, which can degrade the beta-lactam moiety present in penicillins and cephalosporins, and can thus be resistant to those drugs.
Synonyms and brand names
- Synonyms
- Cefalotina
- Cefalotine
- Cefalotinum
- Cefalothin
- Cephalothin Sodium
- Cephalothinum
- Cephalothin
- Cephalotin
- CLS
- Brand Names
- Averon-1®
- Cemastin®
- Coaxin®
- Keflin®
- Seffin®
External links
The most up-to-date information can be obtained at the following sites.
- Cefalotin - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Template:MedMaster
- Template:DrugBank