Oseltamivir: Difference between revisions
Jump to navigation
Jump to search

imported>David E. Volk (chem infobox) |
imported>David E. Volk mNo edit summary |
||
| Line 3: | Line 3: | ||
{{Chem infobox | {{Chem infobox | ||
|align=right | |align=right | ||
|image=[[Image:Oseltamivir structure.jpg|center|thumb| | |image=[[Image:Oseltamivir structure.jpg|center|thumb|350px]] | ||
|width= | |width=350px | ||
|molname=oseltamivir | |molname=oseltamivir | ||
|synonyms= '''Tamiflu®''' | |synonyms= '''Tamiflu®''' | ||
Revision as of 11:48, 24 March 2008
|
| |||||||
| oseltamivir | |||||||
| |||||||
| Uses: | influenza A & B | ||||||
| Properties: | sialic acid homolog, neuraminidase inhibitor | ||||||
| Hazards: | see drug interactions | ||||||
| |||||||
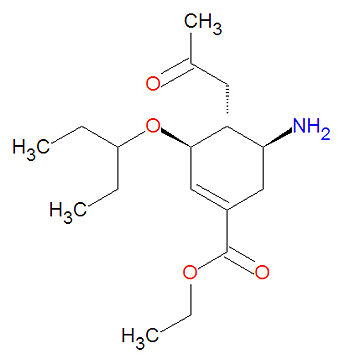
Oseltamivir, or oseltamivir phosphate, marketed as Tamiflu®, is an acetamido cyclohexene that is a structural homolog of sialic acid and it is a neuraminidase inhibitor. It is used to treat influenze A and B infection and also is used prophylactically. The active form is the ester hydrolysed form, a carboxylate. Oseltamivir (like zanamivir) acts as a transition-state analogue inhibitor of influenza neuraminidase.
Chemistry
Its IUPAC chemical name is ethyl (3R,4R,5S)-4-acetamido-5-amino-3-pentan-3-yloxycyclohexene-1-carboxylate and its chemical formula is C16H28N2O4, giving it a molecular mass of 312.4045 g/mol.
External links
- Oseltamivir - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Template:MedMaster
- Template:DrugBank