Ezetimibe: Difference between revisions
imported>David E. Volk m (→External Links) |
imported>Robert Badgett (Started section 'Medical uses') |
||
| Line 3: | Line 3: | ||
'''Ezetimibe''', sold under the brand names '''Ezedoc'''®, '''Zetia'''® and '''Ezetrol'''®, is an anti-[[hyperlipidemic]] medication used to lower [[cholesterol]] levels. It appears to bind to a critical mediator of cholesterol absorption, the Niemann-Pick C1-Like 1 (NPC1L1) protein on the gastrointestinal tract epithelial cells as well as in [[hepatocyte]]s. This mechanism differs from those of other classes of cholesterol-reducing compounds (HMG-CoA reductase inhibitors, bile acid sequestrants, fibric acid derivatives, and plant stanols). Ezetimibe does not inhibit cholesterol synthesis in the liver, or increase bile acid excretion but instead localizes and appears to act at the brush border of the small intestine and inhibits the absorption of cholesterol, leading to a decrease in the delivery of intestinal cholesterol to the liver. This causes a reduction of hepatic cholesterol stores and an increase in clearance of cholesterol from the blood; this distinct mechanism is complementary to that of [[Hydroxymethylglutaryl-coenzyme A reductase inhibitor|HMG-CoA reductase inhibitors]]. | '''Ezetimibe''', sold under the brand names '''Ezedoc'''®, '''Zetia'''® and '''Ezetrol'''®, is an anti-[[hyperlipidemic]] medication used to lower [[cholesterol]] levels. It appears to bind to a critical mediator of cholesterol absorption, the Niemann-Pick C1-Like 1 (NPC1L1) protein on the gastrointestinal tract epithelial cells as well as in [[hepatocyte]]s. This mechanism differs from those of other classes of cholesterol-reducing compounds (HMG-CoA reductase inhibitors, bile acid sequestrants, fibric acid derivatives, and plant stanols). Ezetimibe does not inhibit cholesterol synthesis in the liver, or increase bile acid excretion but instead localizes and appears to act at the brush border of the small intestine and inhibits the absorption of cholesterol, leading to a decrease in the delivery of intestinal cholesterol to the liver. This causes a reduction of hepatic cholesterol stores and an increase in clearance of cholesterol from the blood; this distinct mechanism is complementary to that of [[Hydroxymethylglutaryl-coenzyme A reductase inhibitor|HMG-CoA reductase inhibitors]]. | ||
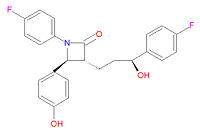
Its chemical IUPAC name is (3R,4S)-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl) azetidin-2-one and its chemical formula is C<sub>24</sub>H<sub>21</sub>F<sub>2</sub>NO<sub>3</sub>. | Its chemical IUPAC name is (3R,4S)-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl) azetidin-2-one and its chemical formula is C<sub>24</sub>H<sub>21</sub>F<sub>2</sub>NO<sub>3</sub>. | ||
| Line 9: | Line 8: | ||
== Drug interactions == | == Drug interactions == | ||
[[Cholestyramine]] decreases the levels of ezetimibe while [[cyclosporine]] increases the effect and toxicity of ezetimibe. Ezetimibe can be taken without regard to food. | [[Cholestyramine]] decreases the levels of ezetimibe while [[cyclosporine]] increases the effect and toxicity of ezetimibe. Ezetimibe can be taken without regard to food. | ||
==Medical uses== | |||
Ezetimibe has been studied in [[randomized controlled trial]]s of treating [[hypercholesterolemia]].<ref name="pmid18376000">{{cite journal |author=Kastelein JJ, Akdim F, Stroes ES, ''et al'' |title=Simvastatin with or without ezetimibe in familial hypercholesterolemia |journal=N. Engl. J. Med. |volume=358 |issue=14 |pages=1431–43 |year=2008 |month=April |pmid=18376000 |doi=10.1056/NEJMoa0800742 |url=http://content.nejm.org/cgi/pmidlookup?view=short&pmid=18376000&promo=ONFLNS19 |issn=}}</ref> | |||
==References== | |||
<references/> | |||
== External Links == | == External Links == | ||
Revision as of 10:21, 12 September 2008
Ezetimibe, sold under the brand names Ezedoc®, Zetia® and Ezetrol®, is an anti-hyperlipidemic medication used to lower cholesterol levels. It appears to bind to a critical mediator of cholesterol absorption, the Niemann-Pick C1-Like 1 (NPC1L1) protein on the gastrointestinal tract epithelial cells as well as in hepatocytes. This mechanism differs from those of other classes of cholesterol-reducing compounds (HMG-CoA reductase inhibitors, bile acid sequestrants, fibric acid derivatives, and plant stanols). Ezetimibe does not inhibit cholesterol synthesis in the liver, or increase bile acid excretion but instead localizes and appears to act at the brush border of the small intestine and inhibits the absorption of cholesterol, leading to a decrease in the delivery of intestinal cholesterol to the liver. This causes a reduction of hepatic cholesterol stores and an increase in clearance of cholesterol from the blood; this distinct mechanism is complementary to that of HMG-CoA reductase inhibitors.
Its chemical IUPAC name is (3R,4S)-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl) azetidin-2-one and its chemical formula is C24H21F2NO3.
Drug interactions
Cholestyramine decreases the levels of ezetimibe while cyclosporine increases the effect and toxicity of ezetimibe. Ezetimibe can be taken without regard to food.
Medical uses
Ezetimibe has been studied in randomized controlled trials of treating hypercholesterolemia.[1]
References
- ↑ Kastelein JJ, Akdim F, Stroes ES, et al (April 2008). "Simvastatin with or without ezetimibe in familial hypercholesterolemia". N. Engl. J. Med. 358 (14): 1431–43. DOI:10.1056/NEJMoa0800742. PMID 18376000. Research Blogging.
External Links
- Ezetimibe - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Template:MedMaster
- Template:DrugBank