Triazole: Difference between revisions
Jump to navigation
Jump to search

imported>David E. Volk (substituted triazole synthesis equations) |
imported>David E. Volk No edit summary |
||
| Line 3: | Line 3: | ||
{{Chem infobox | {{Chem infobox | ||
|align=right | |align=right | ||
|image=[[Image:Triazoles.jpg| | |image=[[Image:Triazoles.jpg|center|thumb|300px]] | ||
|width= | |width=300px | ||
|molname=triazole | |molname=triazole | ||
|synonyms=1,2,3-triazole;1,2,4-triazole | |synonyms=1,2,3-triazole;1,2,4-triazole | ||
|molformula= C<sub>2</sub>H<sub>3</sub>N<sub>3 | |molformula= C<sub>2</sub>H<sub>3</sub>N<sub>3 | ||
|molmass= 69.07 | |molmass= 69.07 | ||
|uses=antifungal | |uses=antifungal | ||
|properties=basic | |properties=basic | ||
|hazards= | |hazards= | ||
|iupac= see | |iupac= see below | ||
|casnumber= 288-88-0; | |casnumber= 288-88-0; | ||
}} | }} | ||
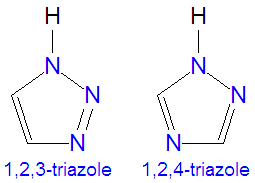
The [[triazole]]s are two isomers, namely 1,2,3-triazole or 1,2,4-triazole, with the formula C<sub>2</sub>H<sub>3</sub>N<sub>3</sub>. They are [[aromatic]] ring compounds that are similar to the [[azole]]s [[pyrazole]] and [[imadazole]], but they would have an additional nitrogen atom in the ring structure. Like the azoles, triazoles are used in many antifungal drugs and fungicides, and the triazole-based drugs are more selective for fungi than mammalian cells compared to the azole-based antifungal compounds. | The [[triazole]]s are two isomers, namely 1,2,3-triazole or 1,2,4-triazole, with the formula C<sub>2</sub>H<sub>3</sub>N<sub>3</sub>. They are [[aromatic]] ring compounds that are similar to the [[azole]]s [[pyrazole]] and [[imadazole]], but they would have an additional nitrogen atom in the ring structure. Like the azoles, triazoles are used in many antifungal drugs and fungicides, and the triazole-based drugs are more selective for fungi than mammalian cells compared to the azole-based antifungal compounds. | ||
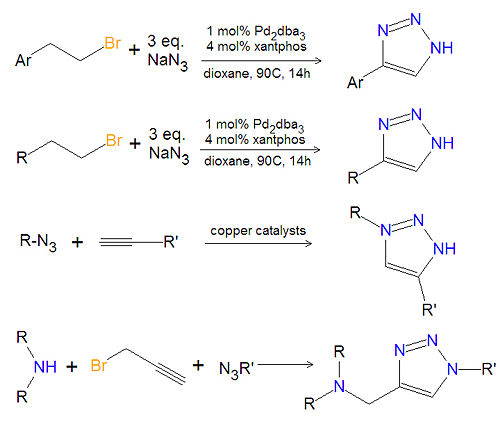
== Chemistry of substituted 1,2,3-triazoles == | |||
[[Image:1,2,3-triazole synthesis substituted.jpg|left|thumb|500px|Triazole synthesis.]] | |||
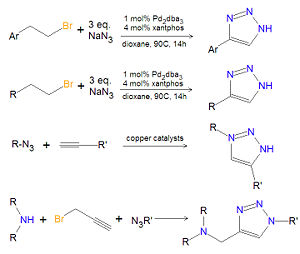
== Chemistry of substituted 1,2,3-triazoles == | == Chemistry of substituted 1,2,3-triazoles == | ||
[[Image:1,2,3-triazole synthesis substituted.jpg|right|thumb|300px|Synthesis of substituted 1,2,3-triazoles.]] | [[Image:1,2,3-triazole synthesis substituted.jpg|right|thumb|300px|Synthesis of substituted 1,2,3-triazoles.]] | ||
Revision as of 09:50, 17 May 2008
|
| |||||||
| triazole | |||||||
| |||||||
| Uses: | antifungal | ||||||
| Properties: | basic | ||||||
| Hazards: | |||||||
| |||||||
The triazoles are two isomers, namely 1,2,3-triazole or 1,2,4-triazole, with the formula C2H3N3. They are aromatic ring compounds that are similar to the azoles pyrazole and imadazole, but they would have an additional nitrogen atom in the ring structure. Like the azoles, triazoles are used in many antifungal drugs and fungicides, and the triazole-based drugs are more selective for fungi than mammalian cells compared to the azole-based antifungal compounds.