Triazole: Difference between revisions
imported>David E. Volk No edit summary |
imported>David E. Volk No edit summary |
||
| Line 16: | Line 16: | ||
}} | }} | ||
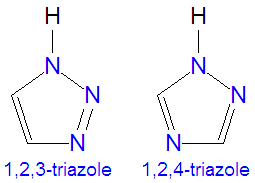
The [[triazole]]s are two isomers, namely 1,2,3-triazole or 1,2,4-triazole, with the formula C<sub>2</sub>H<sub>3</sub>N<sub>3</sub>. They are [[aromatic]] ring compounds that are similar to the [[azole]]s [[pyrazole]] and [[imadazole]], but they would have an additional nitrogen atom in the ring structure. Like the azoles, triazoles are used in many antifungal drugs and fungicides, and the triazole-based drugs are more selective for fungi than mammalian cells compared to the azole-based antifungal compounds. | The [[triazole]]s are two isomers, namely 1,2,3-triazole or 1,2,4-triazole, with the formula C<sub>2</sub>H<sub>3</sub>N<sub>3</sub>. They are [[aromatic]] ring compounds that are similar to the [[azole]]s [[pyrazole]] and [[imadazole]], but they would have an additional nitrogen atom in the ring structure. Like the azoles, triazoles are used in many antifungal drugs and fungicides, and the triazole-based drugs are more selective for fungi than mammalian cells compared to the azole-based antifungal compounds. Today, most substituted triazoles are produced by so-called [[click chemistry]] pioneered by [[K. Narry Sharpless]] and others (see below). | ||
<br><br><br><br><br><br><br><br> | |||
== Chemistry of substituted 1,2,3-triazoles == | == Chemistry of substituted 1,2,3-triazoles == | ||
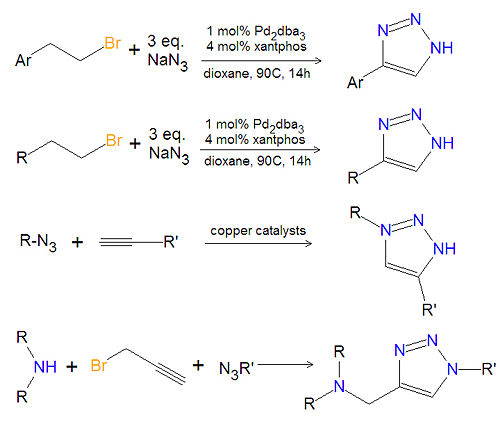
[[Image:1,2,3-triazole synthesis substituted.jpg|left|thumb|500px|Triazole synthesis.]] | [[Image:1,2,3-triazole synthesis substituted.jpg|left|thumb|500px|Triazole synthesis.]] | ||
Most substituted triazoles are synthesized by using one of several click chemistry methods. One method uses paladium catalysts to couple alkenyl halides and sodium azide to product 4-substituted-1,2,3-triazoles<ref>{{cite journal|author=J. Barluenga, C. Valdes, G. Beltran, M. Esribano, and F. Aznar|journal=Angew. Chem. Int. Ed.|year=2006|volume=45|pages=6893-6896}}</ref>. This reaction also works with aromatic alkenyl halides. Click chemistry between azides and terminal aklynes, catalyzed by various copper reagents, can be used to synthesize 3,5-disubstituted-1,2,3-triazoles<ref>{{cite journal|V.V. Rostovtsev, LG Green, VV Fokin and KB Sharpless|journal=Angew. Chem.|volume=114|year=2002|pages=2708-2711}}</ref>,<ref>{{cite journal|author=B.H. Lipshutz, B. R. Taft|journal=Angew. Chem. Int. Ed.|year=2006|volume=45|pages=8235-8238}}</ref>. | |||
== References == | |||
<references/> | |||
Revision as of 11:05, 17 May 2008
|
| |||||||
| triazole | |||||||
| |||||||
| Uses: | antifungal | ||||||
| Properties: | basic | ||||||
| Hazards: | |||||||
| |||||||
The triazoles are two isomers, namely 1,2,3-triazole or 1,2,4-triazole, with the formula C2H3N3. They are aromatic ring compounds that are similar to the azoles pyrazole and imadazole, but they would have an additional nitrogen atom in the ring structure. Like the azoles, triazoles are used in many antifungal drugs and fungicides, and the triazole-based drugs are more selective for fungi than mammalian cells compared to the azole-based antifungal compounds. Today, most substituted triazoles are produced by so-called click chemistry pioneered by K. Narry Sharpless and others (see below).
Chemistry of substituted 1,2,3-triazoles
Most substituted triazoles are synthesized by using one of several click chemistry methods. One method uses paladium catalysts to couple alkenyl halides and sodium azide to product 4-substituted-1,2,3-triazoles[1]. This reaction also works with aromatic alkenyl halides. Click chemistry between azides and terminal aklynes, catalyzed by various copper reagents, can be used to synthesize 3,5-disubstituted-1,2,3-triazoles[2],[3].