Cefalotin: Difference between revisions
imported>David E. Volk mNo edit summary |
imported>Milton Beychok (Undo revision 100471676 by David E. Volk (Talk) Undid inadvertant deletion by David Volk) |
||
| Line 18: | Line 18: | ||
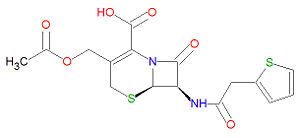
'''Cefalotin''', also called '''sefalothin''', is a semisynthetic first generation [[cephalosporin]] [[antibiotic]] compound that is structurally similar to other cephalosporins including [[cefaclor]], [[cefadroxil]] and [[cefazolin]]. | '''Cefalotin''', also called '''sefalothin''', is a semisynthetic first generation [[cephalosporin]] [[antibiotic]] compound that is structurally similar to other cephalosporins including [[cefaclor]], [[cefadroxil]] and [[cefazolin]]. | ||
It is administered [[parenterally]] to prevent infections during surgery or for treating a broad spectrum of infections of the blood, bone or joints, respiratory tract, skin, and urinary tract. | It is administered [[parenterally]] to prevent infections during surgery or for treating a broad spectrum of infections of the blood, bone or joints, respiratory tract, skin, and urinary tract. | ||
== Chemistry == | |||
The IUPAC chemical name for cefalotin is (6R,7R)-3-(acetyloxymethyl)-8-oxo-7-[(2-thiophen-2-ylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid and it has chemical formula C<sub>16</sub>H<sub>16</sub>N<sub>2</sub>O<sub>6</sub>S<sub>2</sub>, giving it a molecular mass of 396.4380 g/mol. The presence of a [[beta-lactam]] moiety makes it susceptible to degradation by [[beta-lactamase]s present in some bacterial strains. The structure of cefalotin and other cephalosporins resemble those of [[penicillin]]s, but have a six-membered ring attached to the four-membered ring, wherease the penicillins have a five-membered ring attached to the four-membered ring. | |||
== Mechanism of action == | |||
Like penicillin and other cephalosporins, cefalotin works by disrupting the last stage of bacterial cell wall synthesis, leading to [[autolysis]] of the bacteria cells by autolytic enzymes such as the [[autolysin]]s. Some bacteria gain the ability to produce beta-lactamases, which can degrade the beta-lactam moiety present in penicillins and cephalosporins, and can thus be resistant to those drugs. | |||
== Synonyms and brand names == | |||
* '''Synonyms''' | |||
* Cefalotina | |||
* Cefalotine | |||
* Cefalotinum | |||
* Cefalothin | |||
* Cephalothin Sodium | |||
* Cephalothinum | |||
* Cephalothin | |||
* Cephalotin | |||
* CLS | |||
* '''Brand Names''' | |||
* Averon-1® | |||
* Cemastin® | |||
* Coaxin® | |||
* Keflin® | |||
* Seffin® | |||
== External links == | |||
* {{CZMed}} | |||
Revision as of 11:43, 6 April 2009
|
| |||||||
| cefalotin | |||||||
| |||||||
| Uses: | antibiotic drug | ||||||
| Properties: | beta-lactam | ||||||
| Hazards: | see drug interactions | ||||||
| |||||||
Cefalotin, also called sefalothin, is a semisynthetic first generation cephalosporin antibiotic compound that is structurally similar to other cephalosporins including cefaclor, cefadroxil and cefazolin. It is administered parenterally to prevent infections during surgery or for treating a broad spectrum of infections of the blood, bone or joints, respiratory tract, skin, and urinary tract.
Chemistry
The IUPAC chemical name for cefalotin is (6R,7R)-3-(acetyloxymethyl)-8-oxo-7-[(2-thiophen-2-ylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid and it has chemical formula C16H16N2O6S2, giving it a molecular mass of 396.4380 g/mol. The presence of a beta-lactam moiety makes it susceptible to degradation by [[beta-lactamase]s present in some bacterial strains. The structure of cefalotin and other cephalosporins resemble those of penicillins, but have a six-membered ring attached to the four-membered ring, wherease the penicillins have a five-membered ring attached to the four-membered ring.
Mechanism of action
Like penicillin and other cephalosporins, cefalotin works by disrupting the last stage of bacterial cell wall synthesis, leading to autolysis of the bacteria cells by autolytic enzymes such as the autolysins. Some bacteria gain the ability to produce beta-lactamases, which can degrade the beta-lactam moiety present in penicillins and cephalosporins, and can thus be resistant to those drugs.
Synonyms and brand names
- Synonyms
- Cefalotina
- Cefalotine
- Cefalotinum
- Cefalothin
- Cephalothin Sodium
- Cephalothinum
- Cephalothin
- Cephalotin
- CLS
- Brand Names
- Averon-1®
- Cemastin®
- Coaxin®
- Keflin®
- Seffin®
External links
- The most up-to-date information about Cefalotin and other drugs can be found at the following sites.
- Cefalotin - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Cefalotin - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Cefalotin - Detailed information from DrugBank.