Cloxacillin: Difference between revisions
imported>David E. Volk (typos) |
imported>David E. Volk m (brand names) |
||
| Line 2: | Line 2: | ||
[[Image:Cloxacillin structure.jpg|right|thumb|250px|{{#ifexist:Template:Cloxacillin structure.jpg/credit|{{Cloxacillin structure.jpg/credit}}<br/>|}}Cloxacillin]] | [[Image:Cloxacillin structure.jpg|right|thumb|250px|{{#ifexist:Template:Cloxacillin structure.jpg/credit|{{Cloxacillin structure.jpg/credit}}<br/>|}}Cloxacillin]] | ||
'''Cloxacillin''', a chlorinated form of [[oxacillin]], is a [[penicillin]]-like, beta-[[lactam]] [[antibiotic]] used to treat infections from beta-[[lactamase]]-producing ([[penicillase]]-producing) [[staphylocci]], including [[pneumococci]], group A beta-hemolytic streptococci, penicillin G-sensitive and penicillin G-resistant staphylococci.[http://www.drugbank.ca/cgi-bin/getCard.cgi?CARD=DB01147.txt]. | '''Cloxacillin''', a chlorinated form of [[oxacillin]] sold under the brand names '''Novo-Cloxin®''' and | ||
'''Nu-Cloxi®''', is a [[penicillin]]-like, beta-[[lactam]] [[antibiotic]] used to treat infections from beta-[[lactamase]]-producing ([[penicillase]]-producing) [[staphylocci]], including [[pneumococci]], group A beta-hemolytic streptococci, penicillin G-sensitive and penicillin G-resistant staphylococci.[http://www.drugbank.ca/cgi-bin/getCard.cgi?CARD=DB01147.txt]. | |||
== Mechanism of action == | == Mechanism of action == | ||
Revision as of 11:36, 11 February 2008
Cloxacillin, a chlorinated form of oxacillin sold under the brand names Novo-Cloxin® and Nu-Cloxi®, is a penicillin-like, beta-lactam antibiotic used to treat infections from beta-lactamase-producing (penicillase-producing) staphylocci, including pneumococci, group A beta-hemolytic streptococci, penicillin G-sensitive and penicillin G-resistant staphylococci.[1].
Mechanism of action
Like other penicillin-like drugs, cloxacillin interferes with the last stage of bacterial cell wall synthesis by binding to penicillin-binding proteins, thus inhibiting the cross-linking needed to from a stable bacterial cell wall. This leads to autolysis of the bacteria by autolysins.
Chemistry
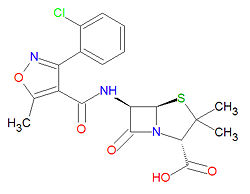
The IUPAC chemical name of cloxacillin is (2S,5R,6R)-6-[[3-(2-chlorophenyl)-5-methyl1,2-oxazole-4-carbonyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid and its chemical formula is C19H18ClN3O5S. It has a beta-lactam structure.
Drug interactions
The drugs demeclocycline , doxycycline, methacycline, minocycline, oxytetracycline, rolitetracycline and tetracycline may be antagonists of cloxacillin. Oral contraceptives, including ethinyl estradiol and mestranol be less effective when taken with cloxacillin. The anticoagulant effects of acenocoumarol, anisindione, dicumarol, methotrexate and warfarin are increased when taken with cloxacillin.
External links
- Cloxacillin - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Drug Bank [2]