Acetaldehyde: Difference between revisions
Jump to navigation
Jump to search

imported>David E. Volk (micro stub and picture) |
imported>David E. Volk No edit summary |
||
| Line 2: | Line 2: | ||
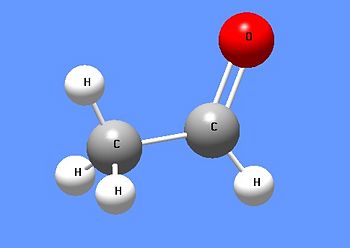
[[Image:Acetaldehyde DEVolk.jpg|right|thumb|350px|{{#ifexist:Template:Acetaldehyde DEVolk.jpg/credit|{{Acetaldehyde DEVolk.jpg/credit}}<br/>|}}Acetaldehyde, the second smallest aldehyde.]] | [[Image:Acetaldehyde DEVolk.jpg|right|thumb|350px|{{#ifexist:Template:Acetaldehyde DEVolk.jpg/credit|{{Acetaldehyde DEVolk.jpg/credit}}<br/>|}}Acetaldehyde, the second smallest aldehyde.]] | ||
'''Acetaldehyde''' is the second smallest [[aldehyde]], second only to [[formaldehyde]]. As an aldehyde, it is a useful chemical for the addition of two carbon atoms to another chemical. | '''Acetaldehyde''' is the second smallest [[aldehyde]], second only to [[formaldehyde]]. As an aldehyde, it is a useful chemical for the addition of two carbon atoms to another chemical. It is produced biosynthetically by the reductive decarboxylation of [[pyruvate]], and it can then be further reduced (by [[NADH]]) to ethanol. However, the reduction of acetaldehyde to ethanol is reversible, so that excess alcohol (ethanol) consumption leads to a build up of acetaldehyde and "hangovers". | ||
Revision as of 18:27, 4 January 2008
Acetaldehyde is the second smallest aldehyde, second only to formaldehyde. As an aldehyde, it is a useful chemical for the addition of two carbon atoms to another chemical. It is produced biosynthetically by the reductive decarboxylation of pyruvate, and it can then be further reduced (by NADH) to ethanol. However, the reduction of acetaldehyde to ethanol is reversible, so that excess alcohol (ethanol) consumption leads to a build up of acetaldehyde and "hangovers".