Acyclovir: Difference between revisions
imported>David E. Volk |
mNo edit summary |
||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
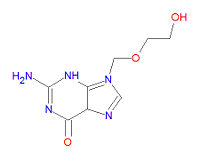
{{Image|Acyclovir structure.jpg|right|200px|Acyclovir.}} | |||
'''Acyclovir''', or '''aciclovir''', is an antimetabolite drug used to treat viral infections. It is an analog of the DNA base [[guanosine]] that is particularly used to treat and manage herpes zoster (shingles), genital herpes, and chickenpox infections. It is the prototype antiviral agent that is activated by viral thymidine kinase. The selective activity of aciclovir is due to its affinity for the thymidine kinase enzyme encoded by HSV and HZV. The drug is converted to the triphosphate version, which competes with the natural deoxyguanosine triphosphate for incorporation into viral DNA. Once incorporated, aciclovir triphosphate inhibits DNA synthesis by acting as a chain terminator. Aciclovir may cause [[nephrotoxicity]] and [[neurotoxicity]] but symptoms usually resolve after cessation of treatment. | '''Acyclovir''', or '''aciclovir''', is an antimetabolite drug used to treat viral infections. It is an analog of the DNA base [[guanosine]] that is particularly used to treat and manage herpes zoster (shingles), genital herpes, and chickenpox infections. It is the prototype antiviral agent that is activated by viral thymidine kinase. The selective activity of aciclovir is due to its affinity for the thymidine kinase enzyme encoded by HSV and HZV. The drug is converted to the triphosphate version, which competes with the natural deoxyguanosine triphosphate for incorporation into viral DNA. Once incorporated, aciclovir triphosphate inhibits DNA synthesis by acting as a chain terminator. Aciclovir may cause [[nephrotoxicity]] and [[neurotoxicity]] but symptoms usually resolve after cessation of treatment. | ||
| Line 6: | Line 6: | ||
Its IUPAC chemical name is 2-amino-9-(2-hydroxyethoxymethyl)-3H-purin-6-one and its chemical formula is | Its IUPAC chemical name is 2-amino-9-(2-hydroxyethoxymethyl)-3H-purin-6-one and its chemical formula is | ||
C<sub>8</sub>H<sub>11</sub>N<sub>5</sub>O<sub>3</sub>. | C<sub>8</sub>H<sub>11</sub>N<sub>5</sub>O<sub>3</sub>. | ||
== Synonyms and Brand names == | == Synonyms and Brand names == | ||
| Line 27: | Line 26: | ||
*Zovirax | *Zovirax | ||
== References == | {{CZMed}} | ||
==References== | |||
{{reflist}} | |||
[[Category:Reviewed Passed]][[Category:Suggestion Bot Tag]] | |||
Latest revision as of 12:00, 6 July 2024
Acyclovir, or aciclovir, is an antimetabolite drug used to treat viral infections. It is an analog of the DNA base guanosine that is particularly used to treat and manage herpes zoster (shingles), genital herpes, and chickenpox infections. It is the prototype antiviral agent that is activated by viral thymidine kinase. The selective activity of aciclovir is due to its affinity for the thymidine kinase enzyme encoded by HSV and HZV. The drug is converted to the triphosphate version, which competes with the natural deoxyguanosine triphosphate for incorporation into viral DNA. Once incorporated, aciclovir triphosphate inhibits DNA synthesis by acting as a chain terminator. Aciclovir may cause nephrotoxicity and neurotoxicity but symptoms usually resolve after cessation of treatment.
Its IUPAC chemical name is 2-amino-9-(2-hydroxyethoxymethyl)-3H-purin-6-one and its chemical formula is C8H11N5O3.
Synonyms and Brand names
Synonyms
- Wellcome-248u
- Acycloguanosine
- Acyclovir Sodium
- AC2
- Aciclovier
- Acyclovir
- Aciclovir Sodium
- 9-Hydroxyethoxymethylguanine
Brand names
- Alti-Acyclovir
- Avirax
- Vipral
- Virorax
- Zovir
- Zovirax
The most up-to-date information about Acyclovir and other drugs can be found at the following sites.
- Acyclovir - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Acyclovir - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Acyclovir - Detailed information from DrugBank.