Cystine: Difference between revisions
Jump to navigation
Jump to search

imported>David E. Volk (stub) |
mNo edit summary |
||
| (4 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
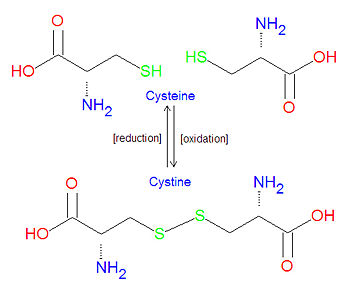
'''Cystine''' refers to the oxidative linkage between two molecules of cysteine, one of the common amino | {{Image|Cysteine vs Cystine10.jpg|right|350px|Comparison of cysteine and cystine structures, which can be interconverted by oxidation or reduction.}} | ||
'''Cystine''' refers to the oxidative linkage between two molecules of [[cysteine]], one of the common [[amino acid]]s, in the form of a [[disulfide bond]]. It may also refer to such a bond that occurs between two cysteine amino acids in a [[protein]]. Typically, intracellular proteins have few, if any, such disulfide bonds, while extracellular proteins tend to have several of them. | |||
Such disulfide bonds are integral to the structural stability of many proteins.[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 16:01, 3 August 2024
Cystine refers to the oxidative linkage between two molecules of cysteine, one of the common amino acids, in the form of a disulfide bond. It may also refer to such a bond that occurs between two cysteine amino acids in a protein. Typically, intracellular proteins have few, if any, such disulfide bonds, while extracellular proteins tend to have several of them.
Such disulfide bonds are integral to the structural stability of many proteins.