Phosphorus: Difference between revisions
imported>Paul Wormer |
mNo edit summary |
||
| (60 intermediate revisions by 17 users not shown) | |||
| Line 2: | Line 2: | ||
{{Elem_Infobox | {{Elem_Infobox | ||
|elName=Phosphorus | |elName=Phosphorus | ||
|eltrnCfg=1s<sup>2</sup>2s<sup>2</sup>2p<sup>6</sup>3s<sup>2</sup>3p<sup>3</sup> | |||
|elgroup=15 | |||
|elperiod=3 | |||
|eltrnCfg= | |elblock=p | ||
|no1= +3 | |no1= +3 | ||
|no2= +5 | |no2= +5 | ||
| Line 15: | Line 13: | ||
|properties=allotropic | |properties=allotropic | ||
|compounds=Oxides, oxyacids, halides | |compounds=Oxides, oxyacids, halides | ||
|uses=Vital to life; | |uses=Vital to life; fertilizers; pesticides; acids; drying agents | ||
|hazard= flammable, toxic | |hazard= flammable, toxic | ||
}} | }} | ||
'''Phosphorus''' is a | '''Phosphorus''' is a [[Chemical elements|chemical element]]. Unlike other elements in group VA of the [[periodic table of elements|periodic table]], phosphorus is never found as a pure element in nature, but only in combination with other elements. Elemental phosphorus is a [[Solid_(state_of_matter)|solid]]. It has the [[chemical symbol]] P, [[atomic number]] (number of [[protons]]) ''Z'' = 15, and a [[Atomic mass#Standard atomic weights of the elements|standard atomic weight]] of 30.973761 g/mol. | ||
in the | |||
It is present in all living organisms in the form of [[organophosphate]]s and as calcium phosphates such as [[hydroxyapatite]] (Ca<sub>10</sub>(PO<sub>4</sub>)<sub>6</sub>(OH)<sub>2</sub>) and [[fluoroapatite]] (Ca<sub>10</sub>(PO<sub>4</sub>)<sub>6</sub>F<sub>2</sub>), substances found in teeth and bones. Many cell signaling cascades in living organisms operate by a series of phosphorylation events in which a phosphate group (PO<sub>4</sub>)<sup>2−</sup> is either added to a protein by a [[kinase]] or removed from a protein by a [[phosphorylase]]. | |||
Both [[red phosphorus]] and [[tetraphosphorus trisulfide]] are used in common matches because they are easily ignited by heat. However, the [[agriculture|agricultural industry]] is the largest user of phosphorus in the form of [[fertilizer]]s. | |||
The radioactive isotope <sup>32</sup>P is used to radiolabel compounds for scientific studies. Phosphorus and arsenic share many chemical properties. | |||
{{TOC|left}} | |||
== Production of elemental phosphorus == | == Production of elemental phosphorus == | ||
Calcium phosphate (phosphate rock), mostly mined in Florida and North Africa, can be heated to 1200-1500 | Calcium phosphate (phosphate rock), mostly mined in Florida and North Africa, can be heated to 1200-1500 Celsius with sand, which is mostly SiO<sub>2</sub>, and coke (impure carbon) to produce vaporized tetraphosphorus, P<sub>4</sub>, (mp. 44.2 C) which is subsequently condensed into a white power under water to prevent oxidation. Even under water, [[white phosphorus]] is slowly converted to the more stable red phosphorus [[allotrope]] (mp. 597C). Both the white and red allotropes of phosphorus are insoluble in water. | ||
== Fertilizers == | == Fertilizers == | ||
Due to the essential nature of phosphorus to living organisms, the low solubility of natural phosphorus-containing compounds, and the slow natural cycle of phosphorous, the agricultural industry is heavily reliant on fertilizers which contain phosphate, mostly in the form of [[superphosphate of lime]]. Superphosphate of lime is a mixture of two phosphate salts, calcium | Due to the essential nature of phosphorus to living organisms, the low solubility of natural phosphorus-containing compounds, and the slow natural cycle of phosphorous, the agricultural industry is heavily reliant on fertilizers which contain phosphate, mostly in the form of [[superphosphate of lime]]. Superphosphate of lime is a mixture of two phosphate salts, calcium dihydrogen phosphate Ca(H<sub>2</sub>PO<sub>4</sub>)<sub>2</sub> and calcium sulfate dihydrate CaSO<sub>4</sub>•2H<sub>2</sub>O produced by the reaction of sulfuric acid and water with calcium phosphate. | ||
== Allotropes of phosphorus == | == Allotropes of phosphorus == | ||
{{Image|Phosphorus P4 atomic structure.jpg|right|200px|Structure of white phosphorus.}} | |||
Both phosphorus and arsenic have many [[allotrope]]s, but only the white and red forms predominate. | Both phosphorus and arsenic have many [[allotrope]]s, but only the white and red forms predominate. | ||
*White phosphorus and yellow arsenic both have four atoms arranged in a tetrahedral structure in which each atom is bound to the other three atoms by a single bond. This form of the elements are the least stable, most reactive, more volatile, less dense, and more toxic than the other allotropes. The toxicity of white phosphorus | *White phosphorus and yellow arsenic both have four atoms arranged in a tetrahedral structure in which each atom is bound to the other three atoms by a single bond. This form of the elements are the least stable, most reactive, more volatile, less [[Density (chemistry)|dense]], and more toxic than the other allotropes. The toxicity of white phosphorus led to its discontinued use in matches. The crystal melts at 44 <sup>0</sup>C and has a density of 1.83 kg/L. The liquid boils at 280 <sup>0</sup>C. | ||
*Red phosphorus: here one of the bonds in P<sub>4</sub> described above has been broken, and one additional bond is formed with a neighboring tetrahedron. | *Red phosphorus: here one of the bonds in P<sub>4</sub> described above has been broken, and one additional bond is formed with a neighboring tetrahedron. | ||
*Black phosphorus is made of even larger aggregates. P<sub>2</sub>, | *Black phosphorus is made of even larger aggregates and is the least reactive allotrope. It is also known as β-metallic phosphorus and has a structure somewhat resembling that of [[graphite]]. | ||
* The diphosphorus allotrope, P<sub>2</sub>, is stable only at high temperatures. The dimeric unit contains a triple bond and is analogous to N<sub>2</sub>. | |||
*Violet phosphorus (also known as [[Hittorf]]'s or α-metallic phosphorus) is obtained by crystallization from molten [[lead]]. Its density (2.34 kg/L) is higher than that of white phosphorus. | |||
==Chemical bonding== | ==Chemical bonding== | ||
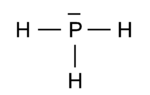
The phosphorus cation is very similar to the nitrogen cation. In the same way | {{Image|PH3.png|right|150px|Fig. A. Phosphine}} | ||
Because phosphorus is just below [[nitrogen]] in the [[Periodic table of elements|periodic table]], the two elements share many of their bonding characteristics. For instance, phosphine, PH<sub>3</sub>, is an analogue of ammonia, NH<sub>3</sub>. Phosphorus, like nitrogen, is trivalent in this molecule. Figure A shows the pre-quantum mechanical [[Lewis structure]], which although somewhat of a simplification from a quantum chemical point of view,<ref>In quantum chemical valence bond theory one usually works with mixtures of ''s'' and ''p'' [[atomic orbital]]s, so-called [[hybridization|hybrids]]</ref> illustrates some of the | |||
distinguishing characteristics. The unpaired electrons in the three 3''p'' orbitals bind with those in the hydrogen 1''s'' orbitals to form electron pairs of opposite [[spin]]. The electron pair in the phosphorus 3''s'' orbital shows up as a lone pair in the Lewis structure. | |||
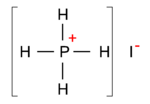
{{Image|Phosphonium iodide.png|right|150px|Fig. B. Phosphonium iodide}} | |||
The phosphorus chloride molecule is an example, | The phosphorus [[cation]] is very similar to the nitrogen cation. In the same way that nitrogen forms the tetravalent ammonium ion, phosphorus can form the tetravalent phosphonium ion, and form salts such as phosphonium iodide [PH<sub>4</sub>]<sup>+</sup>[I<sup>−</sup>], see Fig. B. | ||
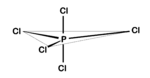
With strongly electronegative ions, in particular fluorine, hexavalency as in PF<sub>6</sub><sup>−</sup> occurs as well. This octahedral ion is [[isoelectronic]] with SF<sub>6</sub>. | Like other elements in the third or lower rows of the periodic table, phosphorus atoms can expand their valence to make penta- and hexavalent compounds. The phosphorus chloride molecule is an example, see Fig. C. When the phosphorus ligands are not identical, the more electronegative ligands are located in the apical positions and the least electronegative ligands are located in the axial positions. | ||
{{Image|PCl5.png|right|150px|Fig. C. Phosphorus chloride}} | |||
With strongly electronegative ions, in particular fluorine, hexavalency as in PF<sub>6</sub><sup>−</sup> occurs as well. This octahedral ion is [[isoelectronic]] with SF<sub>6</sub>. According to the bonding theory of [[Linus Pauling|Pauling]]<ref>L. Pauling,''The Nature of the Chemical Bond'', 3rd edition, Cornell University Press, Ithaca NY (1960)</ref> the six octahedral ''sp''<sup>3</sup>''d''<sup>2</sup> hybrid atomic orbitals play an important role. | |||
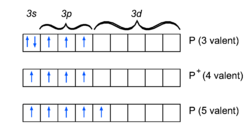
{{Image|Valence states of phosphorus.png|left|250px|Fig. D. Valence states of phosphorus, diagram after Ref. <ref>J. A. A. Ketelaar, ''Chemical Constitution'', Elsevier, Amsterdam (1958), p. 166 </ref>}} | |||
Before extensive computer calculations were feasible, it was generally assumed, following Pauling, that the nearby ''d'' orbitals in the ''n'' = 3 shell (see Fig. D) were the obvious cause of the difference in binding between nitrogen and phosphorus. However, in the early eighties the German theoretical chemist [[Werner Kutzelnigg]]<ref>W. Kutzelnigg, ''Chemical Bonding in Higher Main Group Elements'', Angewandte Chemie Int. (English) Ed., vol. '''23''', pp. 272-295 (1984)</ref> found from an analysis of computer calculations that the difference in binding is more likely due to differences in character between the valence 2''p'' and valence 3''p'' orbitals of nitrogen and phosphorus, respectively. The 2''s'' and 2''p'' orbitals of first row atoms are localized in roughly the same region of space, while the 3''p'' orbitals of phosphorus are much more extended in space than the 3''s'' orbitals. The violation of the octet rule observed in compounds of phosphorus is then due to the size of the phosphorus atom and the corresponding reduction of steric hindrance between its ligands. In modern theoretical chemistry, Kutzelnigg's analysis is generally accepted. | |||
== Phosphine, diphosphine and phosphonium salts == | == Phosphine, diphosphine and phosphonium salts == | ||
Phosphine (PH<sub>3</sub>) and arsine (AsH<sub>3</sub>) are structural analogs with ammonia (NH<sub>3</sub>) and form pyramidal structures with the phosphorus or arsenic atom in the center bound to three hydrogen atoms and one lone electron pair. Both are colorless, ill-smelling, toxic compounds. Phosphine is produced in a manner similar to the production of ammonia. Hydrolysis of calcium phosphide, Ca<sub>3</sub>P<sub>2</sub>, or calcium nitride, Ca<sub>3</sub>N<sub>2</sub> produce phosphine or ammonia, respectively. Unlike ammonia, phosphine is unstable and it reacts instantly with air giving off phosphoric acid clouds. Arsenic is even less stable. Although phosphine is less basic than ammonia, it can form | Phosphine (PH<sub>3</sub>) and arsine (AsH<sub>3</sub>) are structural analogs with ammonia (NH<sub>3</sub>) and form pyramidal structures with the phosphorus or arsenic atom in the center bound to three hydrogen atoms and one lone electron pair. Both are colorless, ill-smelling, toxic compounds. Phosphine is produced in a manner similar to the production of ammonia. Hydrolysis of calcium phosphide, Ca<sub>3</sub>P<sub>2</sub>, or calcium nitride, Ca<sub>3</sub>N<sub>2</sub> produce phosphine or ammonia, respectively. Unlike ammonia, phosphine is unstable and it reacts instantly with air giving off phosphoric acid clouds. Arsenic is even less stable. Although phosphine is less basic than ammonia, it can form some phosphonium salts (like PH<sub>4</sub>I), analogs of ammonium salts, but these salts immediately decompose in water and do not yield phosphonium (PH<sub>4</sub><sup>+</sup>) ions. Diphosphine (P<sub>2</sub>H<sub>4</sub> or H<sub>2</sub>P-PH<sub>2</sub>) is an analog of [[hydrazine]] (N<sub>2</sub>H<sub>4</sub>) that is a colorless liquid which spontaneously ignites in air and can disproportionate into phosphine and complex hydrides. | ||
== Phosphorus halides == | == Phosphorus halides == | ||
| Line 59: | Line 65: | ||
== Phosphorus oxides (acid anhydrides) == | == Phosphorus oxides (acid anhydrides) == | ||
Phosphorus(III)oxide, P<sub>4</sub>O<sub>6</sub> (also called tetraphosphorus hexoxide) and phosphorus(IV) oxide, P<sub>4</sub>O<sub>10</sub> (or tetraphosphorus decoxide) are acid anhydrides of phosphorus oxyacids and hence readily react with water. P<sub>4</sub>O<sub>10</sub> is a particularly good dehydrating agent that can even remove water from nitric acid, HNO<sub>3</sub>. The structure of P<sub>4</sub>O<sub>6</sub> is like that of P<sub>4</sub> with an oxygen atom inserted between each of the P-P bonds. The structure of P<sub>4</sub>O<sub>10</sub> is like that of P<sub>4</sub>O<sub>6</sub> with the addition of one oxygen bond to each phosphorus atom via a double bond and protruding away from the tetrahedral structure. | Phosphorus(III) oxide, P<sub>4</sub>O<sub>6</sub> (also called tetraphosphorus hexoxide) and phosphorus(IV) oxide, P<sub>4</sub>O<sub>10</sub> (or tetraphosphorus decoxide) are acid anhydrides of phosphorus oxyacids and hence readily react with water. P<sub>4</sub>O<sub>10</sub> is a particularly good dehydrating agent that can even remove water from [[nitric acid]], HNO<sub>3</sub>. The structure of P<sub>4</sub>O<sub>6</sub> is like that of P<sub>4</sub> with an oxygen atom inserted between each of the P-P bonds. The structure of P<sub>4</sub>O<sub>10</sub> is like that of P<sub>4</sub>O<sub>6</sub> with the addition of one oxygen bond to each phosphorus atom via a double bond and protruding away from the tetrahedral structure. | ||
== Phosphorus oxyacids == | == Phosphorus oxyacids == | ||
| Line 84: | Line 90: | ||
</table> | </table> | ||
==Widely used phosphorous compounds== | ==Widely used phosphorous compounds== | ||
<br> | |||
<table border="1" cellpadding="2" cellspacing="0" bordercolor="#CCCCCC" bgcolor="#FFFFFF"> | <table border="1" cellpadding="2" cellspacing="0" bordercolor="#CCCCCC" bgcolor="#FFFFFF"> | ||
<tr><th>Phosphorous compound</th><th>Use</th></tr> | <tr><th>Phosphorous compound</th><th>Use</th></tr> | ||
| Line 95: | Line 103: | ||
<tr><td>P<sub>4</sub>S<sub>10</sub></td><td>Manufacturing of additives and pesticides</td></tr> | <tr><td>P<sub>4</sub>S<sub>10</sub></td><td>Manufacturing of additives and pesticides</td></tr> | ||
<tr><td>Na<sub>5</sub>P<sub>3</sub>O<sub>10</sub></td><td>Detergents</td></tr> | <tr><td>Na<sub>5</sub>P<sub>3</sub>O<sub>10</sub></td><td>Detergents</td></tr> | ||
</table> | </table><br> | ||
== Isotopes of phosphorus == | == Isotopes of phosphorus == | ||
Although twenty three isotopes of phosphorus are known (all | Although twenty three isotopes of phosphorus are known <ref>The Berkeley Laboratory Isotopes Project [http://ie.lbl.gov/education/parent/P_iso.htm]</ref> (all possibilities from <sup>24</sup>P up to <sup>46</sup>P), only <sup>31</sup>P, with spin 1/2, is stable and is therefore present at 100% abundance. The half-integer spin and high abundance of <sup>31</sup>P make it useful for nuclear magnetic resonance studies of biomolecules, particularly DNA. | ||
Two radioactive isotopes of phosphorous have half-lives which make them useful for scientific experiments. <sup>32</sup>P has a half-life of 14.262 days and <sup>33</sup>P has a half-life of 25.34 days. Biomolecules can be "tagged" with a radio isotope to allow for the study of very dilute samples. | |||
== Biological membranes and [[phospholipid|phospholipids]] == | == Biological membranes and [[phospholipid|phospholipids]] == | ||
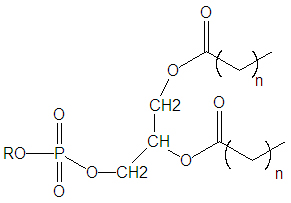
{{Image|Phospholipid DEVolk.jpg|right|350px|Structure of a generic saturated phospholipid. Often n=10,12,14,16,18 and 22.}} | |||
All cells must have a membrane that distinguishes it from the cell's surrounding. Biological membranes are made from a phospholipid matrix and proteins, typically in the form of a bilayer. Phospholipids are derived from [[glycerol]], such that two of the glycerol hydroxyl (OH) protons have been replaced with fatty acids as an [[ester]], and the third hydroxyl proton has been replaced with phosphate bonded to another alcohol. | All cells must have a membrane that distinguishes it from the cell's surrounding. Biological membranes are made from a phospholipid matrix and proteins, typically in the form of a bilayer. Phospholipids are derived from [[glycerol]], such that two of the glycerol hydroxyl (OH) protons have been replaced with fatty acids as an [[ester]], and the third hydroxyl proton has been replaced with phosphate bonded to another alcohol. | ||
==History== | ==History== | ||
Phosphorus was discovered by the Hamburg alchemist Hennig Brand | Phosphorus was discovered by the Hamburg alchemist Hennig Brand in 1669. He made it out of human urine. He let urine stand for days until it gave off a terrible smell. Then he boiled it down to a paste, heated this paste to a high temperature, and led the vapors through water where he hoped they would condense to gold. Instead, he obtained a white, waxy substance that glowed in the dark. Brand had discovered phosphorus, the first element discovered since antiquity. The word phosphorus comes from the Greek (φως = light, φορέω = carry) and means light carrier. We now know that Brand produced ammonium sodium hydrogen phosphate (NH<sub>4</sub>)NaHPO<sub>4</sub>. | ||
Brand at first tried to keep the method secret, but later sold the recipe for 200 thaler to somebody from Dresden, Krafft, who could now make it as well, and toured much of Europe with it, including England, where he met with [[Robert Boyle]]. The secret that it was made from urine leaked out and first Johann Kunckel (1630-1703) in Sweden (1678) and later Boyle in London (1680) also managed to make phosphorus. Boyle states that Krafft gave him no no information as to the preparation of phosphorus other than that it was derived from "somewhat that belonged to the body of man". This gave Boyle a valuable clue, however, so that he, too, managed to make phosphorus. Later he improved Brand's process by using sand in the reaction (still using urine as base material), | Brand at first tried to keep the method secret,<ref>J. M. Stillman, ''The Story of Alchemy and Early Chemistry'', Dover, New York (1960), pp. 418-419</ref> but later sold the recipe for 200 thaler to somebody from Dresden, Krafft, who could now make it as well, and toured much of Europe with it, including England, where he met with [[Robert Boyle]]. The secret that it was made from urine leaked out and first Johann Kunckel (1630-1703) in Sweden (1678) and later Boyle in London (1680) also managed to make phosphorus. Boyle states that Krafft gave him no no information as to the preparation of phosphorus other than that it was derived from "somewhat that belonged to the body of man". This gave Boyle a valuable clue, however, so that he, too, managed to make phosphorus. Later he improved Brand's process by using sand in the reaction (still using urine as base material), | ||
: 4NaPO<sub>3</sub> + 2SiO<sub>2</sub> + 10C → 2Na<sub>2</sub>SiO<sub>3</sub> + 10CO + P<sub>4</sub> | : 4NaPO<sub>3</sub> + 2SiO<sub>2</sub> + 10C → 2Na<sub>2</sub>SiO<sub>3</sub> + 10CO + P<sub>4</sub> | ||
| Line 116: | Line 123: | ||
In 1769 [[Johan Gottlieb Gahn]] and [[Carl Wilhelm Scheele]] showed that calcium phosphate (Ca<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub>) is found in bones and they obtained phosphorus from bone ash. [[Antoine Lavoisier]] recognized phosphorus as an element in 1777. Bone ash was the major source of phosphorus until the 1840s. Phosphate rock, a mineral containing calcium phosphate, was first used in 1850 and following the introduction of the electric arc furnace in 1890 this became the only source of phosphorus. Phosphorus, phosphates and phosphoric acid are still obtained from phosphate rock. Phosphate rock is a major feedstock in the fertilizer industry. | In 1769 [[Johan Gottlieb Gahn]] and [[Carl Wilhelm Scheele]] showed that calcium phosphate (Ca<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub>) is found in bones and they obtained phosphorus from bone ash. [[Antoine Lavoisier]] recognized phosphorus as an element in 1777. Bone ash was the major source of phosphorus until the 1840s. Phosphate rock, a mineral containing calcium phosphate, was first used in 1850 and following the introduction of the electric arc furnace in 1890 this became the only source of phosphorus. Phosphorus, phosphates and phosphoric acid are still obtained from phosphate rock. Phosphate rock is a major feedstock in the fertilizer industry. | ||
==Glow of phosphorus== | ==Glow of phosphorus== | ||
In the dark pure white phosphorus glows, which explains the name of the element. This glow greatly fascinated the discoverers of the element. The phenomenon [[phosphorescence]] has been named after it. Phosphorescence is the slow decay of a metastable electronic state to a lower state under emission of light. The decay is slow because the transition from the excited to the lower state is forbidden, usually because it requires a spin flip. Often it involves a transition from an excited spin-triplet state to a ground state that is a spin-singlet. The metastable excited state may have been populated by thermal excitations or some light source. Since phosphorescence is slow, it persists for some time after the exciting source is removed. | In the dark pure white phosphorus glows, which explains the name of the element. This greenish glow greatly fascinated the discoverers of the element. The phenomenon [[phosphorescence]] has been named after it. Phosphorescence is the slow decay of a metastable electronic state to a lower state under emission of light. The decay is slow because the transition from the excited to the lower state is forbidden, usually because it requires a spin flip. Often it involves a transition from an excited spin-triplet state to a ground state that is a spin-singlet. The metastable excited state may have been populated by thermal excitations or some light source. Since phosphorescence is slow, it persists for some time after the exciting source is removed. | ||
It is only natural to assume that the name giver exhibits the phenomenon named after it. However, this is not the case. The glow of pure phosphorus is due to another phenomenon, namely [[chemiluminescence]]. This occurs when some product molecules of a chemical reaction leave the reaction in an electronically excited state. When the transition from the excited state to the ground state is forbidden, decay of the product molecule(s) will be slow. | It is only natural to assume that the name giver exhibits the phenomenon named after it. However, this is not the case. The glow of pure phosphorus is due to another phenomenon, namely [[chemiluminescence]]. This occurs when some product molecules of a chemical reaction leave the reaction in an electronically excited state. When the transition from the excited state to the ground state is forbidden, decay of the product molecule(s) will be slow. The frequency (color) of the light emitted is proportional to the energy difference of the two electronic states involved, as [[Niels Bohr]] discovered in the early days of quantum mechanics (1913). | ||
Robert Boyle already knew that air was required to make phosphorus glow, so in fact it has been clear since its discovery that phosphorus does not | Robert Boyle already knew that air was required to make phosphorus glow, so in fact it has been clear since its discovery that phosphorus does not phosphoresce but chemiluminesce. However, the exact chemical reaction that caused it was not known until 1974<ref>R. J. van Zee and A. U. Khan, Journal American Chemical Society, vol. '''96''', p. 6805 (1974)</ref> when it was shown that | ||
the excited molecule HPO and the dissociating excited dimer (PO)<sub>2</sub> are the originators of the green glow. The molecules HPO and PO are formed by the oxidization of white phosphorus in the presence of air and water. | the excited molecule HPO and the dissociating excited dimer (PO)<sub>2</sub> are the originators of the green glow. The molecules HPO and PO are formed by the oxidization of white phosphorus in the presence of air and water. | ||
==Note and | ==Note and references== | ||
<references /> | <references />[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 16:01, 3 October 2024
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Phosphorus is a chemical element. Unlike other elements in group VA of the periodic table, phosphorus is never found as a pure element in nature, but only in combination with other elements. Elemental phosphorus is a solid. It has the chemical symbol P, atomic number (number of protons) Z = 15, and a standard atomic weight of 30.973761 g/mol.
It is present in all living organisms in the form of organophosphates and as calcium phosphates such as hydroxyapatite (Ca10(PO4)6(OH)2) and fluoroapatite (Ca10(PO4)6F2), substances found in teeth and bones. Many cell signaling cascades in living organisms operate by a series of phosphorylation events in which a phosphate group (PO4)2− is either added to a protein by a kinase or removed from a protein by a phosphorylase.
Both red phosphorus and tetraphosphorus trisulfide are used in common matches because they are easily ignited by heat. However, the agricultural industry is the largest user of phosphorus in the form of fertilizers.
The radioactive isotope 32P is used to radiolabel compounds for scientific studies. Phosphorus and arsenic share many chemical properties.
Production of elemental phosphorus
Calcium phosphate (phosphate rock), mostly mined in Florida and North Africa, can be heated to 1200-1500 Celsius with sand, which is mostly SiO2, and coke (impure carbon) to produce vaporized tetraphosphorus, P4, (mp. 44.2 C) which is subsequently condensed into a white power under water to prevent oxidation. Even under water, white phosphorus is slowly converted to the more stable red phosphorus allotrope (mp. 597C). Both the white and red allotropes of phosphorus are insoluble in water.
Fertilizers
Due to the essential nature of phosphorus to living organisms, the low solubility of natural phosphorus-containing compounds, and the slow natural cycle of phosphorous, the agricultural industry is heavily reliant on fertilizers which contain phosphate, mostly in the form of superphosphate of lime. Superphosphate of lime is a mixture of two phosphate salts, calcium dihydrogen phosphate Ca(H2PO4)2 and calcium sulfate dihydrate CaSO4•2H2O produced by the reaction of sulfuric acid and water with calcium phosphate.
Allotropes of phosphorus
Both phosphorus and arsenic have many allotropes, but only the white and red forms predominate.
- White phosphorus and yellow arsenic both have four atoms arranged in a tetrahedral structure in which each atom is bound to the other three atoms by a single bond. This form of the elements are the least stable, most reactive, more volatile, less dense, and more toxic than the other allotropes. The toxicity of white phosphorus led to its discontinued use in matches. The crystal melts at 44 0C and has a density of 1.83 kg/L. The liquid boils at 280 0C.
- Red phosphorus: here one of the bonds in P4 described above has been broken, and one additional bond is formed with a neighboring tetrahedron.
- Black phosphorus is made of even larger aggregates and is the least reactive allotrope. It is also known as β-metallic phosphorus and has a structure somewhat resembling that of graphite.
- The diphosphorus allotrope, P2, is stable only at high temperatures. The dimeric unit contains a triple bond and is analogous to N2.
- Violet phosphorus (also known as Hittorf's or α-metallic phosphorus) is obtained by crystallization from molten lead. Its density (2.34 kg/L) is higher than that of white phosphorus.
Chemical bonding
Because phosphorus is just below nitrogen in the periodic table, the two elements share many of their bonding characteristics. For instance, phosphine, PH3, is an analogue of ammonia, NH3. Phosphorus, like nitrogen, is trivalent in this molecule. Figure A shows the pre-quantum mechanical Lewis structure, which although somewhat of a simplification from a quantum chemical point of view,[1] illustrates some of the distinguishing characteristics. The unpaired electrons in the three 3p orbitals bind with those in the hydrogen 1s orbitals to form electron pairs of opposite spin. The electron pair in the phosphorus 3s orbital shows up as a lone pair in the Lewis structure.
The phosphorus cation is very similar to the nitrogen cation. In the same way that nitrogen forms the tetravalent ammonium ion, phosphorus can form the tetravalent phosphonium ion, and form salts such as phosphonium iodide [PH4]+[I−], see Fig. B.
Like other elements in the third or lower rows of the periodic table, phosphorus atoms can expand their valence to make penta- and hexavalent compounds. The phosphorus chloride molecule is an example, see Fig. C. When the phosphorus ligands are not identical, the more electronegative ligands are located in the apical positions and the least electronegative ligands are located in the axial positions.
With strongly electronegative ions, in particular fluorine, hexavalency as in PF6− occurs as well. This octahedral ion is isoelectronic with SF6. According to the bonding theory of Pauling[2] the six octahedral sp3d2 hybrid atomic orbitals play an important role.
Before extensive computer calculations were feasible, it was generally assumed, following Pauling, that the nearby d orbitals in the n = 3 shell (see Fig. D) were the obvious cause of the difference in binding between nitrogen and phosphorus. However, in the early eighties the German theoretical chemist Werner Kutzelnigg[4] found from an analysis of computer calculations that the difference in binding is more likely due to differences in character between the valence 2p and valence 3p orbitals of nitrogen and phosphorus, respectively. The 2s and 2p orbitals of first row atoms are localized in roughly the same region of space, while the 3p orbitals of phosphorus are much more extended in space than the 3s orbitals. The violation of the octet rule observed in compounds of phosphorus is then due to the size of the phosphorus atom and the corresponding reduction of steric hindrance between its ligands. In modern theoretical chemistry, Kutzelnigg's analysis is generally accepted.
Phosphine, diphosphine and phosphonium salts
Phosphine (PH3) and arsine (AsH3) are structural analogs with ammonia (NH3) and form pyramidal structures with the phosphorus or arsenic atom in the center bound to three hydrogen atoms and one lone electron pair. Both are colorless, ill-smelling, toxic compounds. Phosphine is produced in a manner similar to the production of ammonia. Hydrolysis of calcium phosphide, Ca3P2, or calcium nitride, Ca3N2 produce phosphine or ammonia, respectively. Unlike ammonia, phosphine is unstable and it reacts instantly with air giving off phosphoric acid clouds. Arsenic is even less stable. Although phosphine is less basic than ammonia, it can form some phosphonium salts (like PH4I), analogs of ammonium salts, but these salts immediately decompose in water and do not yield phosphonium (PH4+) ions. Diphosphine (P2H4 or H2P-PH2) is an analog of hydrazine (N2H4) that is a colorless liquid which spontaneously ignites in air and can disproportionate into phosphine and complex hydrides.
Phosphorus halides
The trihalides PF3, PCl3, PBr3 and PI3 and the pentahalides PF5, PCl5 and PBr5 are all known and mixed halides can also be formed. The trihalides can be formed simply by mixing the appropriate stoichiometric amounts of phosphorus and a halide. For safety reasons, however, PF3 is typically made by reacting PCl3 with AsF5 and fractional distillation because the direct reaction of phosphorus with fluorine can be explosive. The pentahalides, PX5, are synthesized by reacting excess halide with either elemental phosphorus or with the corresponding trihalide. Mixed phosphorus halides are unstable and decompose to form simple halides. Thus 5PF3Br2 decomposes into 3PF5 and 2PBr5.
Phosphorus oxides (acid anhydrides)
Phosphorus(III) oxide, P4O6 (also called tetraphosphorus hexoxide) and phosphorus(IV) oxide, P4O10 (or tetraphosphorus decoxide) are acid anhydrides of phosphorus oxyacids and hence readily react with water. P4O10 is a particularly good dehydrating agent that can even remove water from nitric acid, HNO3. The structure of P4O6 is like that of P4 with an oxygen atom inserted between each of the P-P bonds. The structure of P4O10 is like that of P4O6 with the addition of one oxygen bond to each phosphorus atom via a double bond and protruding away from the tetrahedral structure.
Phosphorus oxyacids
Phosphorous oxyacids can have acidic protons bound to oxygen atoms and nonacidic protons which are bonded directly to the phosphorus atom. Although many oxyacids of phosphorus are formed, only six are important (see table), and three of them, hypophosphorous acid, phosphorous acid and phosphoric acid are particularly important ones.
| Oxidation State | Formula | Name | Acidic Protons | Compounds |
|---|---|---|---|---|
| +1 | H3PO2 | hypophosphorous acid | 1 | acid, salts |
| +3 | H3PO3 | (ortho)phosphorous acid | 2 | acid, salts |
| +5 | (HPO3)n | metaphosphoric acids | n | salts (n=3,4) |
| +5 | H5P3O10 | triphosphoric acid | 3 | salts |
| +5 | H4P2O7 | pyrophosphoric acid | 4 | acid, salts |
| +5 | H3PO4 | (ortho)phosphoric acid | 3 | acid, salts |
Widely used phosphorous compounds
| Phosphorous compound | Use |
|---|---|
| Ca(H2PO4)2•H2O | Baking powder & fertilizers |
| CaHPO4•2H2O | Animal food additive, toothpowder |
| H3PO4 | Manufacture of phosphate fertilizers |
| PCl3 | Manufacture of POCl3 and pesticides |
| POCl3 | plasticizer Manufacturing |
| P4S10 | Manufacturing of additives and pesticides |
| Na5P3O10 | Detergents |
Isotopes of phosphorus
Although twenty three isotopes of phosphorus are known [5] (all possibilities from 24P up to 46P), only 31P, with spin 1/2, is stable and is therefore present at 100% abundance. The half-integer spin and high abundance of 31P make it useful for nuclear magnetic resonance studies of biomolecules, particularly DNA.
Two radioactive isotopes of phosphorous have half-lives which make them useful for scientific experiments. 32P has a half-life of 14.262 days and 33P has a half-life of 25.34 days. Biomolecules can be "tagged" with a radio isotope to allow for the study of very dilute samples.
Biological membranes and phospholipids
All cells must have a membrane that distinguishes it from the cell's surrounding. Biological membranes are made from a phospholipid matrix and proteins, typically in the form of a bilayer. Phospholipids are derived from glycerol, such that two of the glycerol hydroxyl (OH) protons have been replaced with fatty acids as an ester, and the third hydroxyl proton has been replaced with phosphate bonded to another alcohol.
History
Phosphorus was discovered by the Hamburg alchemist Hennig Brand in 1669. He made it out of human urine. He let urine stand for days until it gave off a terrible smell. Then he boiled it down to a paste, heated this paste to a high temperature, and led the vapors through water where he hoped they would condense to gold. Instead, he obtained a white, waxy substance that glowed in the dark. Brand had discovered phosphorus, the first element discovered since antiquity. The word phosphorus comes from the Greek (φως = light, φορέω = carry) and means light carrier. We now know that Brand produced ammonium sodium hydrogen phosphate (NH4)NaHPO4.
Brand at first tried to keep the method secret,[6] but later sold the recipe for 200 thaler to somebody from Dresden, Krafft, who could now make it as well, and toured much of Europe with it, including England, where he met with Robert Boyle. The secret that it was made from urine leaked out and first Johann Kunckel (1630-1703) in Sweden (1678) and later Boyle in London (1680) also managed to make phosphorus. Boyle states that Krafft gave him no no information as to the preparation of phosphorus other than that it was derived from "somewhat that belonged to the body of man". This gave Boyle a valuable clue, however, so that he, too, managed to make phosphorus. Later he improved Brand's process by using sand in the reaction (still using urine as base material),
- 4NaPO3 + 2SiO2 + 10C → 2Na2SiO3 + 10CO + P4
Robert Boyle was the first to use phosphorus to ignite sulphur-tipped wooden splints, forerunners of our modern matches, in 1680.
In 1769 Johan Gottlieb Gahn and Carl Wilhelm Scheele showed that calcium phosphate (Ca3(PO4)2) is found in bones and they obtained phosphorus from bone ash. Antoine Lavoisier recognized phosphorus as an element in 1777. Bone ash was the major source of phosphorus until the 1840s. Phosphate rock, a mineral containing calcium phosphate, was first used in 1850 and following the introduction of the electric arc furnace in 1890 this became the only source of phosphorus. Phosphorus, phosphates and phosphoric acid are still obtained from phosphate rock. Phosphate rock is a major feedstock in the fertilizer industry.
Glow of phosphorus
In the dark pure white phosphorus glows, which explains the name of the element. This greenish glow greatly fascinated the discoverers of the element. The phenomenon phosphorescence has been named after it. Phosphorescence is the slow decay of a metastable electronic state to a lower state under emission of light. The decay is slow because the transition from the excited to the lower state is forbidden, usually because it requires a spin flip. Often it involves a transition from an excited spin-triplet state to a ground state that is a spin-singlet. The metastable excited state may have been populated by thermal excitations or some light source. Since phosphorescence is slow, it persists for some time after the exciting source is removed.
It is only natural to assume that the name giver exhibits the phenomenon named after it. However, this is not the case. The glow of pure phosphorus is due to another phenomenon, namely chemiluminescence. This occurs when some product molecules of a chemical reaction leave the reaction in an electronically excited state. When the transition from the excited state to the ground state is forbidden, decay of the product molecule(s) will be slow. The frequency (color) of the light emitted is proportional to the energy difference of the two electronic states involved, as Niels Bohr discovered in the early days of quantum mechanics (1913).
Robert Boyle already knew that air was required to make phosphorus glow, so in fact it has been clear since its discovery that phosphorus does not phosphoresce but chemiluminesce. However, the exact chemical reaction that caused it was not known until 1974[7] when it was shown that the excited molecule HPO and the dissociating excited dimer (PO)2 are the originators of the green glow. The molecules HPO and PO are formed by the oxidization of white phosphorus in the presence of air and water.
Note and references
- ↑ In quantum chemical valence bond theory one usually works with mixtures of s and p atomic orbitals, so-called hybrids
- ↑ L. Pauling,The Nature of the Chemical Bond, 3rd edition, Cornell University Press, Ithaca NY (1960)
- ↑ J. A. A. Ketelaar, Chemical Constitution, Elsevier, Amsterdam (1958), p. 166
- ↑ W. Kutzelnigg, Chemical Bonding in Higher Main Group Elements, Angewandte Chemie Int. (English) Ed., vol. 23, pp. 272-295 (1984)
- ↑ The Berkeley Laboratory Isotopes Project [1]
- ↑ J. M. Stillman, The Story of Alchemy and Early Chemistry, Dover, New York (1960), pp. 418-419
- ↑ R. J. van Zee and A. U. Khan, Journal American Chemical Society, vol. 96, p. 6805 (1974)