Carbodiimide: Difference between revisions
Jump to navigation
Jump to search

imported>David E. Volk (New page: <noinclude>{{subpages}}</noinclude> {{Chem infobox |align=right |image=center|thumb|250px |width=250px |molname=carbodiimide |synonyms= |molformula= R1-N=C=N-...) |
imported>David E. Volk No edit summary |
||
| Line 17: | Line 17: | ||
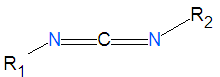
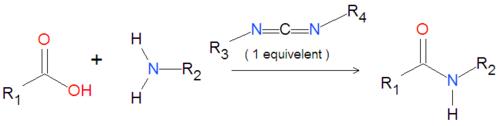
'''Carbodiimides''' are a type of dehydrating chemical most often used to activate [[carboxylic acid]]s for subsequent coupling with primary [[ | '''Carbodiimides''' are a type of dehydrating chemical most often used to activate [[carboxylic acid]]s for subsequent coupling with primary [[amine]]s, producing an [[amide]] compound. the carboxyl group is often converted to an activated compound by forming an [[NHS-ester]]. | ||
{{Image|Carbodiimide generic reaction.png|right|500px|Coupling of an amide group to a carboxylic acid activated by a carbodiimide.}} | |||
Revision as of 09:00, 3 October 2009
|
| |||||||
| carbodiimide | |||||||
| |||||||
| Uses: | dehydration reagent | ||||||
| Properties: | activates carboxylates | ||||||
| Hazards: | |||||||
| |||||||
Carbodiimides are a type of dehydrating chemical most often used to activate carboxylic acids for subsequent coupling with primary amines, producing an amide compound. the carboxyl group is often converted to an activated compound by forming an NHS-ester.