Malaria: Difference between revisions
imported>Sandy Harris (→Prevention: typo) |
imported>Howard C. Berkowitz (→Vector) |
||
| (6 intermediate revisions by the same user not shown) | |||
| Line 9: | Line 9: | ||
*More than one million people die of malaria every year, mostly infants, young children and pregnant women and most of them in Africa | *More than one million people die of malaria every year, mostly infants, young children and pregnant women and most of them in Africa | ||
WHO, in its 2008 report on malaria, in no way minimizes the problem, but sees several areas of distinct progress. Some, however, are already showing difficulties." Half of the world's population is at risk of malaria, and | WHO, in its 2008 report on malaria, in no way minimizes the problem, but sees several areas of distinct progress. Some, however, are already showing difficulties." Half of the world's population is at risk of malaria. | ||
For many years, the rough estimate of a million deaths per year was the conventional wisdom, but a major study in 2001, updated in 2004, indicates the [[#mortality and morbidity of malaria|Mortality and morbidity of malaria]] is worse than that.<ref name=Breman2004>{{citation | |||
| journal = Am J Trop Med Hyg | |||
| title = The Intolerable Burden of Malaria: What's New, What's Needed | |||
| author = Breman JG, Alilio MS, Mills A | |||
| year = 2004 | |||
| url = http://www.ajtmh.org/cgi/content/full/71/2_suppl/0-i | |||
| volume = 71 (2 Supplement) page = i | |||
}}</ref> Three organizations guided the project: | |||
*[[Multilateral Initiative on Malaria]] (MIM), which began in 1997 and aims "to strengthen and sustain, through collaborative research and training, the capability of malaria-endemic countries in Africa to carry out research required to develop and improve tools for malaria control" and to strengthen the research-control interface | |||
*[[Disease Control Priorities in Developing Countries Project]] (DCPP) was launched in 2002 "to assess disease control priorities and produce science-based analyses and resource materials to inform health policy-making in developing countries" to decrease morbidity, mortality, disability, and their economic consequences. | |||
*[[Fogarty International Center]] (FIC), [[National Institutes of Health]] (NIH), was a MIM founding partner, and has led, housed, and fully supported the coordinating Secretariats for MIM (1999 to 2002) and DCPP (2002 to the present). The FIC "promotes and supports scientific discovery internationally and mobilizes resources to reduce disparities in global health." | |||
Funding came from | |||
*Multilateral Initiative on Malaria | |||
*[[Academy for Educational Development]] | |||
*[[Bill and Melinda Gates Foundation]] | |||
*[[Burroughs Wellcome Fund]] | |||
*[[Rockefeller Foundation]] | |||
*[[Swiss Agency for Development and Cooperation]] | |||
*[[United Nations Foundation]] | |||
*U.S. government | |||
**[[Centers for Disease Control]] | |||
**[[National Institutes of Health]] | |||
***Fogarty International Center | |||
*** Foundation for the National Institutes of Health | |||
***National Institute of Allergy and Infectious Diseases | |||
***National Institute of Environmental Health Sciences, | |||
***[[National Library of Medicine]] | |||
***Malaria Vaccine Initiative at the [[Program for Appropriate Technologies in Health]] (PATH) | |||
**[[Agency for International Development]] | |||
*[[Wellcome Trust]] | |||
*[[World Bank]]. | |||
*[[World Health Organization]] (WHO) | |||
**Regional Office for Africa | |||
**Roll Back Malaria Department | |||
**Special Programme for Research and Training in Tropical Diseases | |||
The advent of long-lasting insecticidal nets and [[artemisinin|artemisinin-based combination therapy (ACT)]], plus a revival of support for [[indoor residual spraying of insecticide]], presents a new opportunity for large-scale malaria control. "<ref>{{citation | |||
| author = WHO World Malaria Programme | | author = WHO World Malaria Programme | ||
| url = http://malaria.who.int/wmr2008/ | | url = http://malaria.who.int/wmr2008/ | ||
| Line 16: | Line 54: | ||
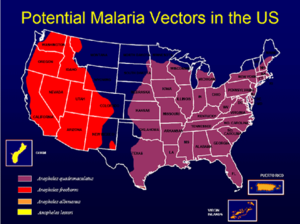
[[Image:Map us malaria vectors.png|thumb|left|Areas where malaria could re-establish in the U.S]] | [[Image:Map us malaria vectors.png|thumb|left|Areas where malaria could re-establish in the U.S]] | ||
Developed countries cannot be complacent. It has been assessed as a world threat in an [[U.S. intelligence and global health|U.S. National Intelligence Estimate]]. | Developed countries cannot be complacent. It has been assessed as a world threat in an [[U.S. intelligence and global health|U.S. National Intelligence Estimate]]. | ||
==Etiology== | |||
The protozoa, of the genus ''Plasmodium'', are carried in the saliva of ''Anopheles'' [[mosquito|mosquitoes]], which reproduce in stagnant water. There are four species: ''P. falciparum, P. vivax, P. ovale,'' and ''P. malariae''. | |||
==Vectors== | |||
Vectors carry the protozoan parasites to the victim. | |||
===Mosquitoes=== | |||
The mosquito vector is present in many parts of the world, and refugees from endemic areas potentially can introduce it, as shown in he U.S. map with areas that have the appropriate mosquitoes. While malaria, in the U.S., there are 1500 cases of malaria annually in the U.S., which could get into the mosquito population.<ref name=CDC-malaria>{{citation | The mosquito vector is present in many parts of the world, and refugees from endemic areas potentially can introduce it, as shown in he U.S. map with areas that have the appropriate mosquitoes. While malaria, in the U.S., there are 1500 cases of malaria annually in the U.S., which could get into the mosquito population.<ref name=CDC-malaria>{{citation | ||
| url = http://www.cdc.gov/malaria/features/refugees.htm | | url = http://www.cdc.gov/malaria/features/refugees.htm | ||
| Line 25: | Line 67: | ||
| date = August 14, 2008 | | date = August 14, 2008 | ||
| title = Malaria in Refugees from Tanzania – King County, Washington, 2007}}</ref> | | title = Malaria in Refugees from Tanzania – King County, Washington, 2007}}</ref> | ||
===Manmade vectors=== | |||
Further, while mosquitoes are the natural vector, malaria certainly can be spread through shared hypodermic needles, either from [[intravenous drug abuse]], or in areas where the healthcare system cannot afford either disposable hypodermics or proper sterilization. Malaria can be transmitted by contaminated blood transfusions. | |||
==Mortality and morbidity of malaria== | |||
Worldwide number of cases are estimated to be 90 million, with 500 million infected people. While figures from 900,000 to 1 million deaths had been used, this excluded Africa partially or completely, certain outside urban centers. 90 percent of cases are believed to be in Africa. " in the 1990s, 26 percent | |||
of the more than 1,500 children born in rural western | |||
Kenya died over a four-year period.<ref>McElroy PD, ter Kuile FO, Hightower AW, Hawley WA, Phillips- | |||
Howard PA, Oloo AJ, Lal AF, Nahlen BL, 2001. ''Am J | |||
Trop Med Hyg'' 64(suppl 1): 18–27. ''quoted by Breman 2004''</ref> Neonatal and infant | |||
mortality were 32 per 1,000 and 176 per 1,000 <ref>Breman 2004, p. iv-vii</ref> | |||
==Prevention== | ==Prevention== | ||
Simple mechanical means are the starting point in controlling them: minimizing standing water and using netting. Appropriate insecticide and insect repellents have a role, although resistance to the main insecticides, [[DDT]] and [[pyrethrins]], is increasing and there are no immediate alternatives. The basic techniques can be enhanced with : | Simple mechanical means are the starting point in controlling them: minimizing standing water and using netting. Appropriate insecticide and insect repellents have a role, although resistance to the main insecticides, [[DDT]] and [[pyrethrins]], is increasing and there are no immediate alternatives. The basic techniques can be enhanced with : | ||
*Indoor Residual Spraying of long-acting insecticide (IRS) | *[[Indoor Residual Spraying]] of long-acting insecticide (IRS) | ||
*Long-Lasting Insecticidal | *[[Long-Lasting Insecticidal Net]]s (LLINs). | ||
Integrated Vector Management (IVM) strategies to kill the developing larvae have to be tailored to each environment. These are long-term activities needing long-term funding and political commitment. | Integrated Vector Management (IVM) strategies to kill the developing larvae have to be tailored to each environment. These are long-term activities needing long-term funding and political commitment. | ||
| Line 55: | Line 103: | ||
Quinine is intensely bitter. Before tablet and capsule dosage forms were common, it had to be drunk. The British, in India, turned an unpleasant task of taking quinine for chemoprophylaxis into a pleasant daily ritual, by incorporating quinine into alcoholic drink mixer "tonics". One's gin-and-tonic was, aside from other attractions, the means of less painfully ingesting one's daily quinine dose. | Quinine is intensely bitter. Before tablet and capsule dosage forms were common, it had to be drunk. The British, in India, turned an unpleasant task of taking quinine for chemoprophylaxis into a pleasant daily ritual, by incorporating quinine into alcoholic drink mixer "tonics". One's gin-and-tonic was, aside from other attractions, the means of less painfully ingesting one's daily quinine dose. | ||
===First-generation | ===First-generation synthetics=== | ||
The first synthetic alternative to quinine was [[quinacrine]], widely known by the trade name of ''Atabrine''. First made in Germany in the 1930s, its distribution to U.S. troops at risk began in 1942. Compliance was a problem, as it turned the skin yellow, and casused nausea, vomiting, diarrhea, skin staining, psychosis, lichen planus, and exfoliative dermatitis. Weina observed that the first dose was often given to troops aboard a ship approaching a combat area, so anxiety, seasickness, and gastrointestinal upset were therefore blamed on the drug when other factors could have been responsible.<ref name=Weina1998/> Military folklore also claimed that quinacrine caused impotence. | The first synthetic alternative to quinine was [[quinacrine]], widely known by the trade name of ''Atabrine''. First made in Germany in the 1930s, its distribution to U.S. troops at risk began in 1942. Compliance was a problem, as it turned the skin yellow, and casused nausea, vomiting, diarrhea, skin staining, psychosis, lichen planus, and exfoliative dermatitis. Weina observed that the first dose was often given to troops aboard a ship approaching a combat area, so anxiety, seasickness, and gastrointestinal upset were therefore blamed on the drug when other factors could have been responsible.<ref name=Weina1998/> Military folklore also claimed that quinacrine caused impotence. | ||
The next major drug, [[chloroquine]] was discovered by a German, Hans Andersag, in 1934 at Bayer I.G. Farbenindustrie A.G. laboratories in Eberfeld, Germany. He named his compound resochin. Through a series of lapses and confusion brought about during the war, chloroquine was finally recognized and established as an effective and safe antimalarial in 1946 by British and U.S. scientists. <ref name=CDC-Malaria-History>{{citation | The next major drug, [[chloroquine]], was discovered by a German, Hans Andersag, in 1934 at Bayer I.G. Farbenindustrie A.G. laboratories in Eberfeld, Germany. He named his compound resochin. Through a series of lapses and confusion brought about during the war, chloroquine was finally recognized and established as an effective and safe antimalarial in 1946 by British and U.S. scientists. <ref name=CDC-Malaria-History>{{citation | ||
| url = http://www.cdc.gov/malaria/history/index.htm | | url = http://www.cdc.gov/malaria/history/index.htm | ||
| title = The History of Malaria, an Ancient Disease | | title = The History of Malaria, an Ancient Disease | ||
| publisher = [[Centers for Disease Control]]}}</ref> Chloroquine has activity against other parasites, and is, with derivatives, a member of the "antimalarial" subclass of [[disease-modifying antirheumatic drugs]]. | | publisher = [[Centers for Disease Control]]}}</ref> Chloroquine has activity against other parasites, and is, with derivatives, a member of the "antimalarial" subclass of [[disease-modifying antirheumatic drugs]]. It may be the single most useful antimalarial drug, although it certainly has its limitations. | ||
By 1947, comparisons were possible, and chloroquine was most preferred. <ref>{{citation | By 1947, comparisons were possible, and chloroquine was most preferred. <ref>{{citation | ||
| Line 67: | Line 115: | ||
| title = Comparison of Chloroquine, Quinacrine (atabrine) and Quinine in the treatment of acute attacks of Sporozite-induced Vivax malaria (Chesson Strain) | | title = Comparison of Chloroquine, Quinacrine (atabrine) and Quinine in the treatment of acute attacks of Sporozite-induced Vivax malaria (Chesson Strain) | ||
| year = 1947 | | year = 1947 | ||
| journal = J. Clinical Invest. | author = Pullman T ''et al.''}}</ref> | | journal = J. Clinical Invest. | author = Pullman T ''et al.''}}</ref> Subsequent comparisons between chloroquine and hydroxychloroquine show the former is more effective, more toxic, and cheaper. | ||
===Antibiotics=== | |||
More general antibiotics, including [[clindamycin]], [[doxycycline]] and [[tetracycline]] have activity. Since their action against active parasites may be slower than that of other drugs, they tend to be preferred for prophylaxis, or in multidrug regimens where their specific modes of action are synergistic with faster-acting drugs. | More general antibiotics, including [[clindamycin]], [[doxycycline]] and [[tetracycline]] have activity. Since their action against active parasites may be slower than that of other drugs, they tend to be preferred for prophylaxis, or in multidrug regimens where their specific modes of action are synergistic with faster-acting drugs. | ||
===Newer agents=== | |||
Approved in 2000 was the fixed-combination, for prophylaxis and treatment, of [[atovaquone-proguanil]]. | Approved in 2000 was the fixed-combination, for prophylaxis and treatment, of [[atovaquone-proguanil]]. | ||
| Line 86: | Line 135: | ||
! | ! | ||
|- | |- | ||
| ''P. falciparum'' | | [[Plasmodium falciparum|''P. falciparum'']] | ||
| Rapidly progressive illness or death | | Rapidly progressive illness or death | ||
| chloroquine or hydroxychloroquine, unless believed resistant; then quinine sulfate plus doxycycline, tetracycline, or clindamycin; '''or''' atovaquone-proguanil (Malarone). | | [[artemisinin|artemisinin-based combination therapy]] '''or''' chloroquine or hydroxychloroquine, unless believed resistant; then quinine sulfate plus doxycycline, tetracycline, or clindamycin; '''or''' atovaquone-proguanil (Malarone). | ||
|- | |- | ||
| ''P. vivax'' | | ''P. vivax'' | ||
| Line 116: | Line 165: | ||
===Artemisinin=== | ===Artemisinin=== | ||
{{main|artemisinin}} | {{main|artemisinin}} | ||
Among the most valuable drugs doday is [[artemisinin]], which was first available as a Chinese herbal preparation. It has been synthesized to help meet demand. To minimize resistance formation, the standard of care defined by the [[World Health Organization]] is '''artemisin combination therapy (ACT)''', either with the original drug or a derivative. Artemisin itself has relatively low bioavailability and has been banned in some countries, to avoid resistance formation due to underdosage | |||
Artesunate is a derivative that produces higher blood levels when given intravenously. In 2007, the U.S. [[Centers for Disease Control]] (CDC) received an exception from the [[Food and Drug Administration]] to Artesunate in the United States, specifically for treatment of malarial clinical emergencies; CDC will dispense it to requesting organizations that meet treatment criteria. | Artesunate is a derivative that produces higher blood levels when given intravenously. In 2007, the U.S. [[Centers for Disease Control]] (CDC) received an exception from the [[Food and Drug Administration]] to Artesunate in the United States, specifically for treatment of malarial clinical emergencies; CDC will dispense it to requesting organizations that meet treatment criteria. | ||
| Line 126: | Line 175: | ||
There is a significant drug counterfeiting problem, which is contributing to resistance. | There is a significant drug counterfeiting problem, which is contributing to resistance. | ||
==References== | ==References== | ||
{{reflist|2}} | {{reflist|2}} | ||
Revision as of 01:31, 27 October 2010
Malaria, caused by four species of Plasmodium protozoa and spread by mosquitoes, causes more deaths, worldwide, than any other vector-borne disease. Coping with malaria must be a two-pronged strategy of preventing and treating the disease in humans, and eradicating the disease-carrying mosquito vectors. The World Health Organization considers it the greatest problem among tropical diseases:[1]
- Malaria is both preventable and curable.
- A child dies of malaria every 30 seconds.
- More than one million people die of malaria every year, mostly infants, young children and pregnant women and most of them in Africa
WHO, in its 2008 report on malaria, in no way minimizes the problem, but sees several areas of distinct progress. Some, however, are already showing difficulties." Half of the world's population is at risk of malaria.
For many years, the rough estimate of a million deaths per year was the conventional wisdom, but a major study in 2001, updated in 2004, indicates the Mortality and morbidity of malaria is worse than that.[2] Three organizations guided the project:
- Multilateral Initiative on Malaria (MIM), which began in 1997 and aims "to strengthen and sustain, through collaborative research and training, the capability of malaria-endemic countries in Africa to carry out research required to develop and improve tools for malaria control" and to strengthen the research-control interface
- Disease Control Priorities in Developing Countries Project (DCPP) was launched in 2002 "to assess disease control priorities and produce science-based analyses and resource materials to inform health policy-making in developing countries" to decrease morbidity, mortality, disability, and their economic consequences.
- Fogarty International Center (FIC), National Institutes of Health (NIH), was a MIM founding partner, and has led, housed, and fully supported the coordinating Secretariats for MIM (1999 to 2002) and DCPP (2002 to the present). The FIC "promotes and supports scientific discovery internationally and mobilizes resources to reduce disparities in global health."
Funding came from
- Multilateral Initiative on Malaria
- Academy for Educational Development
- Bill and Melinda Gates Foundation
- Burroughs Wellcome Fund
- Rockefeller Foundation
- Swiss Agency for Development and Cooperation
- United Nations Foundation
- U.S. government
- Centers for Disease Control
- National Institutes of Health
- Fogarty International Center
- Foundation for the National Institutes of Health
- National Institute of Allergy and Infectious Diseases
- National Institute of Environmental Health Sciences,
- National Library of Medicine
- Malaria Vaccine Initiative at the Program for Appropriate Technologies in Health (PATH)
- Agency for International Development
- Wellcome Trust
- World Bank.
- World Health Organization (WHO)
- Regional Office for Africa
- Roll Back Malaria Department
- Special Programme for Research and Training in Tropical Diseases
The advent of long-lasting insecticidal nets and artemisinin-based combination therapy (ACT), plus a revival of support for indoor residual spraying of insecticide, presents a new opportunity for large-scale malaria control. "[3]
Developed countries cannot be complacent. It has been assessed as a world threat in an U.S. National Intelligence Estimate.
Etiology
The protozoa, of the genus Plasmodium, are carried in the saliva of Anopheles mosquitoes, which reproduce in stagnant water. There are four species: P. falciparum, P. vivax, P. ovale, and P. malariae.
Vectors
Vectors carry the protozoan parasites to the victim.
Mosquitoes
The mosquito vector is present in many parts of the world, and refugees from endemic areas potentially can introduce it, as shown in he U.S. map with areas that have the appropriate mosquitoes. While malaria, in the U.S., there are 1500 cases of malaria annually in the U.S., which could get into the mosquito population.[4] five cases of malaria were diagnosed in 2007, among Burundian refugees who had settled in the state of Washington, in the western United States. [5]
Manmade vectors
Further, while mosquitoes are the natural vector, malaria certainly can be spread through shared hypodermic needles, either from intravenous drug abuse, or in areas where the healthcare system cannot afford either disposable hypodermics or proper sterilization. Malaria can be transmitted by contaminated blood transfusions.
Mortality and morbidity of malaria
Worldwide number of cases are estimated to be 90 million, with 500 million infected people. While figures from 900,000 to 1 million deaths had been used, this excluded Africa partially or completely, certain outside urban centers. 90 percent of cases are believed to be in Africa. " in the 1990s, 26 percent of the more than 1,500 children born in rural western Kenya died over a four-year period.[6] Neonatal and infant mortality were 32 per 1,000 and 176 per 1,000 [7]
Prevention
Simple mechanical means are the starting point in controlling them: minimizing standing water and using netting. Appropriate insecticide and insect repellents have a role, although resistance to the main insecticides, DDT and pyrethrins, is increasing and there are no immediate alternatives. The basic techniques can be enhanced with :
- Indoor Residual Spraying of long-acting insecticide (IRS)
- Long-Lasting Insecticidal Nets (LLINs).
Integrated Vector Management (IVM) strategies to kill the developing larvae have to be tailored to each environment. These are long-term activities needing long-term funding and political commitment.
While there is no vaccine, chemoprophylaxis can be effective, although, as with the use of anti-malarial drugs to treat active infection, drug resistance is an increasing problem.
The Centers for Disease Control recommend [5] that refugees from sub-Saharan Africa routinely be treated, before leaving Africa, with presumptive artemisinin-based combination therapy (ACT; e.g., artemether-lumefantrine).
Treatment
Drug treatment has been challenging. Many of the agents effective against parasites have significant toxicity, and Plasmodium sp. also become resistant. Even recently, major side effects such as increased psychosis became apparent only when a drug went into widespread use. [8]
It is so common, in many areas of Africa, that fever and chills are routinely treated with antimalarial drugs. Misdiagnosis here adds not only to malaria drug resistance, but resistance to other drugs.
In the industrialized world, it is principally a disease of travelers. Clinicians encountering possible malaria must maintain a high index of suspicion, and be sure to take travel histories. Coinfection with other tropical diseases should add suspicion.
Early treatment
Malaria was one of the first drugs with a specific treatment, the alkaloid quinine, from cinchona tree bark, also called Jesuit's Bark. Quinine rarely is a complete cure but suppresses symptoms and is relatively toxic. With the advent of synthetic drugs in the Second World War, it was gradually replaced, although it still has a significant role as a backup drug for resistant forms.
Quinine is intensely bitter. Before tablet and capsule dosage forms were common, it had to be drunk. The British, in India, turned an unpleasant task of taking quinine for chemoprophylaxis into a pleasant daily ritual, by incorporating quinine into alcoholic drink mixer "tonics". One's gin-and-tonic was, aside from other attractions, the means of less painfully ingesting one's daily quinine dose.
First-generation synthetics
The first synthetic alternative to quinine was quinacrine, widely known by the trade name of Atabrine. First made in Germany in the 1930s, its distribution to U.S. troops at risk began in 1942. Compliance was a problem, as it turned the skin yellow, and casused nausea, vomiting, diarrhea, skin staining, psychosis, lichen planus, and exfoliative dermatitis. Weina observed that the first dose was often given to troops aboard a ship approaching a combat area, so anxiety, seasickness, and gastrointestinal upset were therefore blamed on the drug when other factors could have been responsible.[8] Military folklore also claimed that quinacrine caused impotence.
The next major drug, chloroquine, was discovered by a German, Hans Andersag, in 1934 at Bayer I.G. Farbenindustrie A.G. laboratories in Eberfeld, Germany. He named his compound resochin. Through a series of lapses and confusion brought about during the war, chloroquine was finally recognized and established as an effective and safe antimalarial in 1946 by British and U.S. scientists. [9] Chloroquine has activity against other parasites, and is, with derivatives, a member of the "antimalarial" subclass of disease-modifying antirheumatic drugs. It may be the single most useful antimalarial drug, although it certainly has its limitations.
By 1947, comparisons were possible, and chloroquine was most preferred. [10] Subsequent comparisons between chloroquine and hydroxychloroquine show the former is more effective, more toxic, and cheaper.
Antibiotics
More general antibiotics, including clindamycin, doxycycline and tetracycline have activity. Since their action against active parasites may be slower than that of other drugs, they tend to be preferred for prophylaxis, or in multidrug regimens where their specific modes of action are synergistic with faster-acting drugs.
Newer agents
Approved in 2000 was the fixed-combination, for prophylaxis and treatment, of atovaquone-proguanil.
Resistance concerns
Where the disease is not endemic, and the illness is not immediately life-threatening, laboratory diagnosis of the specific Plasmodium species, along with the travel history, will help guide the choice of the appropriate drug treatment. [11] The disease must be reported to local and national public health authorities in non-endemic areas, especially in severe cases where organizations such as the CDC may stock drugs not normally available.
| Plasmodium species | Evaluation | |
|---|---|---|
| P. falciparum | Rapidly progressive illness or death | artemisinin-based combination therapy or chloroquine or hydroxychloroquine, unless believed resistant; then quinine sulfate plus doxycycline, tetracycline, or clindamycin; or atovaquone-proguanil (Malarone). |
| P. vivax | May have dormant state requiring treatment | chloroquine or hydroxychloroquine, unless believed resistant; then quinine sulfate plus doxycycline, tetracycline, or clindamycin; or atovaquone-proguanil (Malarone). |
| P. ovale | May have dormant state requiring treatment | chloroquine or hydroxychloroquine, unless believed resistant; then quinine sulfate plus doxycycline, tetracycline, or clindamycin; or atovaquone-proguanil (Malarone). |
| P. malariae | chloroquine or hydroxychloroquine |
Mild cases can be treated with oral therapy. The presence of any of the following factors may justify urgent parenteral therapy: who have one or more of the following clinical criteria
- impaired consciousness/coma or repeated generalized convulsions
- severe normocytic anemia
- renal failure
- pulmonary edema
- acute respiratory distress syndrome
- circulatory shock
- acidosis,
- disseminated intravascular coagulation or spontaneous bleeding or hemoglobinuria, *jaundice
- blood parasite load of > 5%
Artemisinin
Among the most valuable drugs doday is artemisinin, which was first available as a Chinese herbal preparation. It has been synthesized to help meet demand. To minimize resistance formation, the standard of care defined by the World Health Organization is artemisin combination therapy (ACT), either with the original drug or a derivative. Artemisin itself has relatively low bioavailability and has been banned in some countries, to avoid resistance formation due to underdosage
Artesunate is a derivative that produces higher blood levels when given intravenously. In 2007, the U.S. Centers for Disease Control (CDC) received an exception from the Food and Drug Administration to Artesunate in the United States, specifically for treatment of malarial clinical emergencies; CDC will dispense it to requesting organizations that meet treatment criteria. [12]
There is a significant drug counterfeiting problem, which is contributing to resistance.
References
- ↑ World Health Organization, Malaria
- ↑ Breman JG, Alilio MS, Mills A (2004), "The Intolerable Burden of Malaria: What's New, What's Needed", Am J Trop Med Hyg 71 (2 Supplement) page = i
- ↑ WHO World Malaria Programme, 2008 WORLD MALARIA REPORT, World Health Organization
- ↑ Centers for Disease Control, Malaria
- ↑ 5.0 5.1 "Malaria in Refugees from Tanzania – King County, Washington, 2007", Morbidity and Mortality Weekly Report, August 14, 2008
- ↑ McElroy PD, ter Kuile FO, Hightower AW, Hawley WA, Phillips- Howard PA, Oloo AJ, Lal AF, Nahlen BL, 2001. Am J Trop Med Hyg 64(suppl 1): 18–27. quoted by Breman 2004
- ↑ Breman 2004, p. iv-vii
- ↑ 8.0 8.1 Weina, Peter J (Sep 1998), "From Atabrine in World War II to mefloquine in Somalia: The role of education in preventive medicine", Military Medicine
- ↑ The History of Malaria, an Ancient Disease, Centers for Disease Control
- ↑ Pullman T et al. (1947), "Comparison of Chloroquine, Quinacrine (atabrine) and Quinine in the treatment of acute attacks of Sporozite-induced Vivax malaria (Chesson Strain)", J. Clinical Invest.
- ↑ Centers for Disease Control, Treatment: General Approach § Treatment: Uncomplicated Malaria
- ↑ Jorge Rivas (2 August 2007), "CDC to Distribute Novel Drug for the Treatment of Malaria Emergencies", Associated Content